Flow Cytometry Staining Overview
Immunophenotyping suspended cells based on antigens present on the cell surface is one of the most common uses of flow cytometry. Since cell surface proteins are readily accessible to the antibody, a permeabilization step is not required. By staining cell surface markers, researchers can identify specific cell populations and perform fluorescence-activated cell sorting (FACS).
The following flow cytometry staining protocol has been developed and optimized by R&D Systems Flow Cytometry Laboratory. This protocol is designed for staining of cell surface proteins. It is recommended that experimental conditions, such as antibody concentration, incubation time, and temperature, be optimized for each flow cytometry experiment.
Please read the following flow cytometry staining protocol in its entirety before beginning.

Flow Cytometry Video Protocol
Reagents Required
- Fc Receptor Blocking Reagents (These include Fc receptor blocking antibodies or IgG solutions)
- Flow Cytometry Human Lyse Buffer (10X; Catalog # FC002) or Flow Cytometry Mouse Lyse Buffer (10X; Catalog # FC003) or equivalent
- Flow Cytometry Staining Buffer (Catalog # FC001) or an equivalent solution containing BSA and sodium azide
- Fluorochrome-conjugated antibodies suitable for use in flow cytometry
- Isotype Control Antibodies
Materials Required
- FACS™ Tubes (5 mL round-bottom polystyrene tubes)
- Pipette Tips and Pipettes
- Centrifuge
- Vortex
Sample preparation:
-
For staining peripheral blood cells, whole blood should be collected in evacuated tubes containing EDTA or heparin as the anticoagulant. Contaminating serum components should be removed by washing the cells three times in an isotonic phosphate buffer (supplemented with 0.5% BSA) by centrifugation at 1250-1500 rpm/350-500 x g for 5 minutes.
-
For staining cell lines or cells from activated cell cultures, the cells should be centrifuged at 1250-1500 rpm/350-500 x g for 5 minutes and washed three times in an isotonic PBS buffer (supplemented with 0.5% BSA) to remove any residual growth factors that may be present in the culture medium.
-
Adherent cell lines may require pretreatment with 0.5 mM EDTA to facilitate removal from their substrates. Cells that require trypsinization to enable removal from their substrates should be further incubated in medium for 6-10 hours on a rocker platform to enable regeneration of the receptors. The use of the rocker platform will prevent reattachment to the substrate.
Procedure
- Harvest cells and aliquot up to 1 x 106 cells/100 μL into FACS tubes. Fc-block cells with blocking IgG (1 μg IgG/106 cells) for 15 minutes at room temperature.
Note: Do not wash excess blocking IgG from this reaction. - Add conjugated primary antibody (5-10 μL/106 cells, or a previously titrated amount) and vortex. Incubate cells for 30 minutes at room temperature in the dark.
- Remove any unbound antibody by washing the cells in 2 mL Flow Cytometry Staining Buffer (Catalog # FC001). Centrifuge the suspended cells at 1250-1500 rpm/350-300 x g for 5 minutes and decant the buffer. Resuspend the cells by adding 2 mL of Flow Cytometry Staining Buffer. Repeat this wash step two times.
Note: If using whole blood, samples should go through a red blood cell lysis step at this point. To lyse red blood cells, add 2 mL of 1X Flow Cytometry Human Lyse Buffer (Catalog # FC002) or 1X Flow Cytometry Mouse Lyse Buffer (Catalog # FC003) to each tube. Vortex and incubate in the dark at room temperature for 10 minutes. Centrifuge and wash cells in Flow Cytometry Staining Buffer as described in step 3 above.
Note: If an unconjugated primary antibody is used, incubation with an appropriate secondary antibody should occur now. Dilute the secondary antibody in Flow Cytometry Staining Buffer (Catalog # FC001) starting with the suggested concentration in the product datasheet. Incubate for 20-30 minutes in the dark and perform the wash as described in step 3. - Resuspend cells in 200 – 400 μL of Flow Cytometry Staining Buffer (Catalog # FC001) for final flow cytometric analysis.
It is recommended to run a negative control. A separate set of cells should be stained with an Isotype Control Antibody using the steps outlined above.
FACS is a trademark of Becton Dickinson and Company
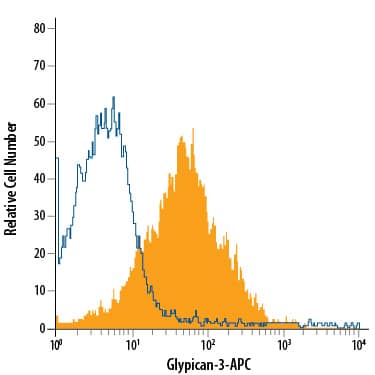
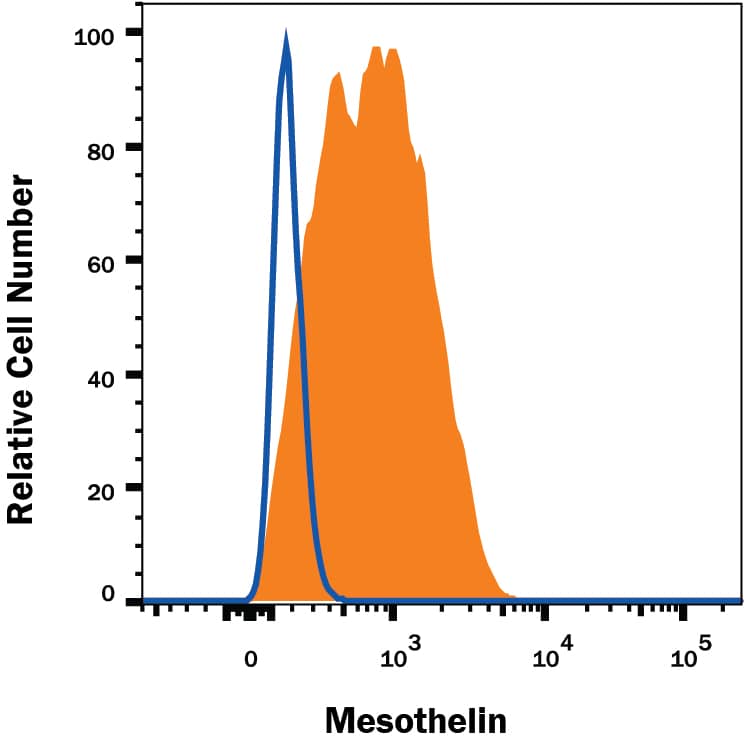
Flow Cytometry Surface Staining Example