Intracellular Flow Cytometry Overview
Flow cytometry can be used to analyze various intracellular molecules including phosphorylated signaling proteins and cytokines. To stain intracellular molecules, the cells need to be fixed in suspension and then permeabilized before the detection antibody is added. This fixation/permeabilization treatment allows the antibody to pass through the plasma membrane into the cell interior, while maintaining the morphological characteristics used to sort the cells. Commonly used detergents include saponin, TritonÆ X-100, or TweenÆ 20.
The following flow cytometry staining protocol has been developed and optimized by R&D Systems Flow Cytometry Laboratory. This protocol is designed for intracellular staining of proteins. It is recommended that experimental conditions, such as antibody concentration, incubation time, and temperature, be optimized for each flow cytometry experiment.
Please read the following flow cytometry staining protocol in its entirety before beginning.

Reagents Required
- PBS (1X): 0.137 M NaCl, 0.05 M NaH2PO4, pH 7.4 or Hank's Balanced Salt Solution (HBSS; 1X)
- Flow Cytometry Fixation Buffer (Catalog # FC004) or an equivalent solution containing 1 - 4% paraformaldehyde
- Flow Cytometry Permeabilization Buffer/Wash Buffer I (Catalog # FC005) or an equivalent solution containing a permeabilization agent such as saponin or Triton X-100
- (Optional, see Note in step 3) Flow Cytometry Fixation/Permeabilization Buffer I (Catalog # FC007)
- Fc Receptor Blocking Reagents (These include Fc receptor blocking antibodies or IgG solutions)
- Fluorochrome-conjugated antibodies suitable for use in flow cytometry
- Isotype Control Antibodies
Materials Required
- FACSô Tubes (5 mL round-bottom polystyrene tubes)
- Pipette Tips and Pipettes
- Centrifuge
- Vortex
Procedure
When performing surface and intracellular staining in the same sample, it is advisable that the staining of cell surface antigens be performed first since fixation and permeabilization treatments might decrease the availability of surface antigens.
- Harvest cells and wash 2 times by adding 2 mL of PBS (or HBSS), centrifuging at 1250-1500 rpm/350-500 x g for 5 minutes, and then decanting buffer from pelleted cells.
- Aliquot up to 1 x 106 cells/100 µL into FACS tubes. Add 0.5 mL of cold Flow Cytometry Fixation Buffer (Catalog # FC004) and vortex. Incubate at room temperature for 10 minutes. Vortex cells intermittently in order to maintain a single cell suspension.
- Centrifuge cells and decant the Fixation Buffer. Wash cells 2 times with PBS (or HBSS) as described in step 1. Resuspend the cell pellet in 100 ñ 200 µL of Flow Cytometry Permeabilization Buffer/Wash Buffer I (Catalog # FC005)
Note: Saponin-mediated cell permeabilization is a reversible process, it is important to keep the cells in the presence of Permeabilization Buffer I during intracellular staining.
Note: Depending on the specific antibody and cell sample being used, the fixation and permeabilization steps can be performed simultaneously using Flow Cytometry Fixation/Permeabilization Buffer I (Catalog # FC007). - Fc-block cells with blocking IgG (1 µg IgG/106 cells) for 15 minutes at room temperature.
Note: Do not wash excess blocking IgG from this reaction. - Add conjugated antibody (5-10 µL/106 cells or a previously titrated amount) and vortex. Incubate cells for 30 minutes at room temperature in the dark.
- Wash cells 2 times with Flow Cytometry Permeabilization Buffer/Wash Buffer I (Catalog # FC005) as described in step 1.
Note: If an unconjugated primary antibody is used, incubation with an appropriate secondary antibody should occur now. Dilute the secondary antibody in PBS (or HBSS), starting with the concentration suggested in the product datasheet. Incubate for 20-30 minutes in the dark and wash as described in step 1. - Resuspend the cells in 200 ñ 400 µL PBS (or HBSS) buffer for flow cytometric analysis.
It is recommended to run a negative control. A separate set of cells should be stained with an Isotype Control Antibody using the steps outlined above.
FACS is a trademark of Becton Dickinson and Company.
Triton is a registered trademark of The Dow Chemical Company.
Tween is a registered trademark of Uniqema Americas LLC.
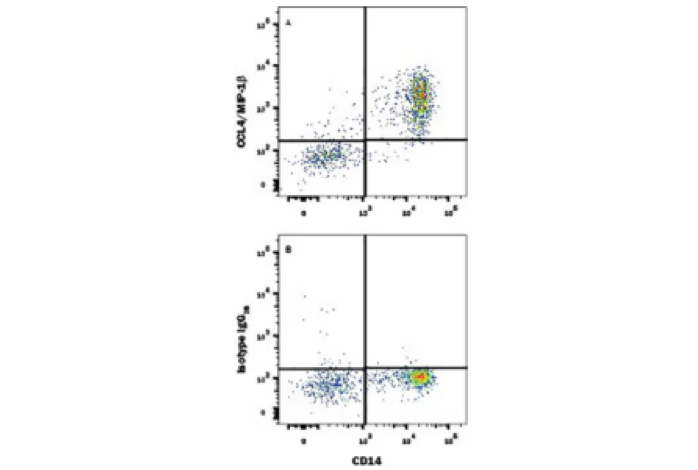
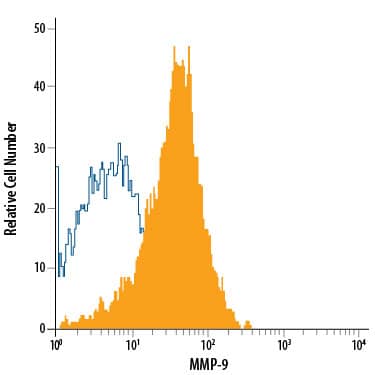
Flow Cytometry Intracellular Staining Example