Enzyme Linked Immunosorbent Assays
Search for ELISA & ELISA Kits |
ELISAs are a type of immunoassay that are commonly used to quantify levels of a specific target within a sample. Samples routinely used in ELISAs include serum, plasma, cell culture supernates, cell lysates, saliva, tissue lysates, and urine.
ELISAs are usually run in 96-well microplates coated with a capture antibody specific for the analyte of interest. Upon incubation with experimental samples, standards, or controls, the target analyte is captured by this antibody. A conjugated detection antibody that binds to a different epitope on the target analyte is used to complete the sandwich. A substrate solution is subsequently added to produce a signal that is proportional to the amount of analyte bound. ELISAs can have different formats.
The four main types of ELISAs
direct | indirect | sandwich | competitive
Each type of ELISA has its own advantages and disadvantages.
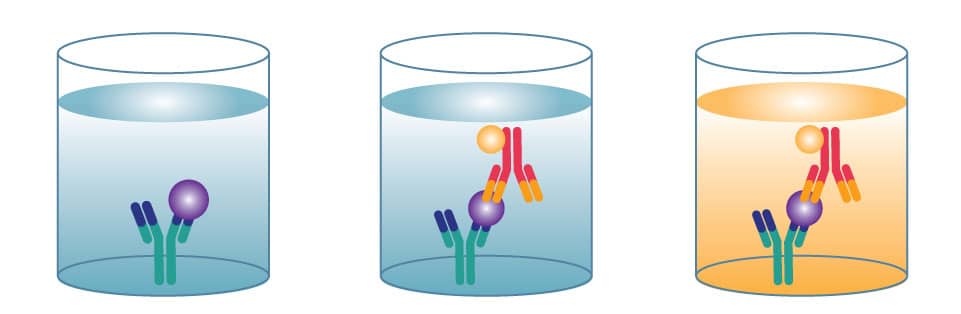
In a direct ELISA, an antigen or sample is immobilized directly on the plate and a conjugated detection antibody binds to the target protein. Substrate is then added, producing a signal that is proportional to the amount of analyte in the sample. Since only one antibody is used in a direct ELISA, they are less specific than a sandwich ELISA.
When to Use: Assessing antibody affinity and specificity. Investigating blocking/inhibitory interactions.
Advantages:
Disadvantages:
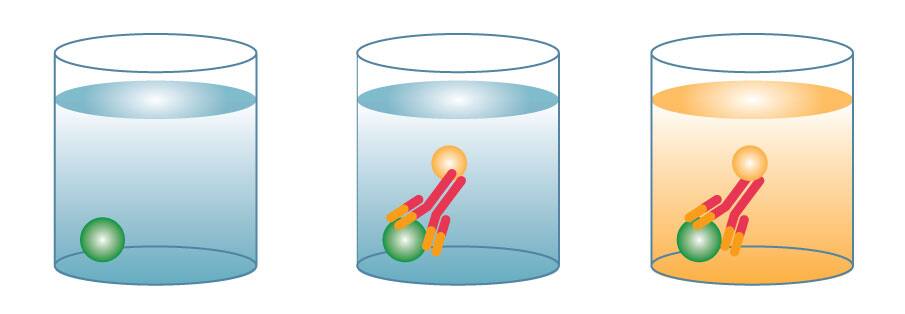
An indirect ELISA is similar to a direct ELISA in that an antigen is immobilized on a plate, but it includes an additional amplification detection step. First, an unconjugated primary detection antibody is added and binds to the specific antigen. A conjugated secondary antibody directed against the host species of the primary antibody is then added. Substrate then produces a signal proportional to the amount of antigen bound in the well.
When to Use: Measuring endogenous antibodies.
Advantages:
Disadvantages:
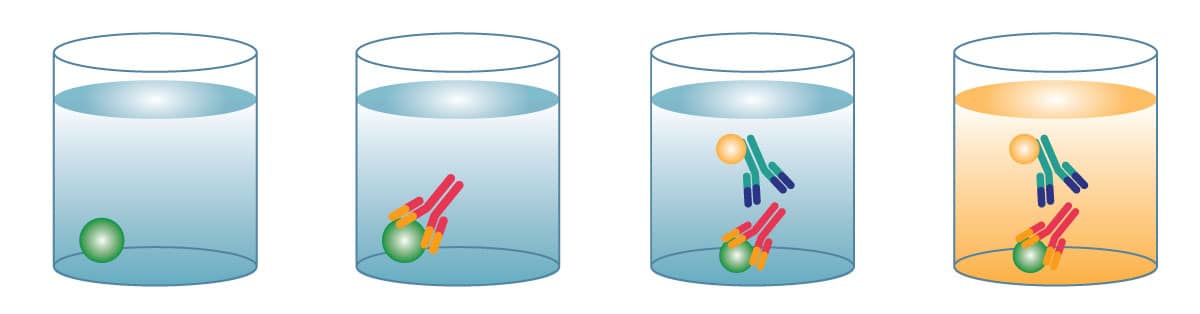
Sandwich ELISAs are the most common type of ELISA. Two specific antibodies are used to sandwich the antigen, commonly referred to as matched antibody pairs. Capture antibody is coated on a microplate, sample is added, and the protein of interest binds and is immobilized on the plate. A conjugated-detection antibody is then added and binds to an additional epitope on the target protein. Substrate is added and produces a signal that is proportional to the amount of analyte present in the sample. Sandwich ELISAs are highly specific, since two antibodies are required to bind to the protein of interest.
When to Use: Determining analyte concentration in a biological sample.
Advantages:
Disadvantages:
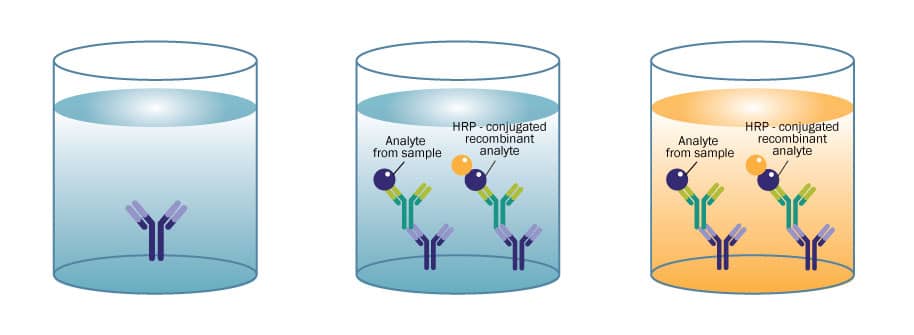
Competitive ELISAs are commonly used for small molecules, when the protein of interest is too small to efficiently sandwich with two antibodies. Similar to a sandwich ELISA, a capture antibody is coated on a microplate. Instead of using a conjugated detection antibody, a conjugated antigen is used to complete for binding with the antigen present in the sample. The more antigen present in the sample, the less conjugated antigen will bind to the capture antibody. Substrate is added and the signal produced is inversely proportional to the amount of protein present in the sample.
When to Use: Determining concentrations of a small molecules and hormones.
Advantages:
Disadvantages:
The difference in a direct vs indirect ELISA is in the detection method of the immobilized antigen on an ELISA plate. Direct ELISAs use a conjugated primary antibody, while indirect ELISAs include an additional amplification step. In an indirect ELISA, an unconjugated primary antibody binds to the antigen, then a labeled secondary antibody directed against the host species of the primary antibody binds to the primary antibody. Indirect ELISAs can be more sensitive than direct ELISA detection methods due to the amplification step, but there is also the risk of cross-reactivity with the antigen, which could cause higher background. Indirect ELISAs also take longer due to the extra step. An advantage to using indirect detection is that you can use the same secondary antibody for multiple different assays, eliminating the need to conjugate primary antibodies for every ELISA.
There are many different immunoassay platforms available to measure to quantitate protein levels in biological fluids. ELISAs are preferred in many cases due to their sensitivity, specificity, accuracy, and ability to tolerate harsh buffers or pretreatments. Comparing an ELISA to a Western blot, sandwich ELISAs use 2 specific antibodies rather than one and allow for completely quantitative results, while a Western blot can see non-specific bands and are semi-quantitative at best. An advantage of ELISAs over different multiplexing platforms is the ability to customize the assay for that antigen and not having to worry about many other antibodies and proteins working together. The potential of observing cross-reactivity or interference is minimized and you can push the sensitivity limits.
ELISAs tend to be the most sensitive immunoassays due to the binding characteristics of the antibodies and the amplification or different read-out systems used. Sample volumes can also be adjusted when you have a very low abundant protein. As discussed above, indirect ELISAs allow for the amplification of signal by using a secondary antibody. Other amplification systems can also be used in ELISAs to make High Sensitivity ELISA Kits, where an additional amplification step is used to increase the sensitivity. Examples of these extra amplification systems are Alkaline Phosphatase or and Streptavidin HPR polymer.