Human PD-1 Antibody Summary
Leu25-Gln167
Accession # Q8IX89
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Human PD-1 Sandwich Immunoassay
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human PD‑1 by Western Blot. Western blot shows lysate of human thymus tissue. PVDF membrane was probed with 2 µg/mL of Goat Anti-Human PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1086) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). Specific bands were detected for PD-1 at approximately 40-50 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Human PD‑1 by Western Blot. Western blot shows lysates of HEK293 human embryonic kidney cell line either mock transfected or transfected with human PD-1. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1086) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). Specific bands were detected for PD-1 at approximately 40-80 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of PD‑1 in Human PBMCs treated with PHA by Flow Cytometry. Human peripheral blood mononuclear cells (PBMCs) either (A) untreated or (B) treated with 5 µg/mL PHA overnight were stained with Goat Anti-Human PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1086) followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107) and Mouse Anti-Human CD3e APC-conjugated Monoclonal Antibody (Catalog # FAB100A). Quadrant markers were set based on control antibody staining (Catalog # F0107). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
PD‑1 in Human Lymph Node. PD-1 was detected in immersion fixed paraffin-embedded sections of human lymph node using Goat Anti-Human PD-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1086) at 3 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Goat HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS008) and counterstained with hematoxylin (blue). Specific staining was localized to lymphocytes. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human PD-1 by Immunohistochemistry PD-L1, PD-L2, PD-1, CD8, and CD4 expression in p16-positive and p16-negative HNSCC. PD-L1, PD-L2, PD-1, CD8, and CD4 expression was assessed in tumor biopsy tissue from five p16-positive and four p16-negative HNSCC patients using immuno-histochemistry (details in Methods). Representative staining (scale bars, 100 μm) and cumulative data of marker expression (grading scale PD-L1/PD-L2: 1, low; 2, moderate; 3 high expression; grading scale PD-1, CD8, CD4: 1, <50 cells/field; 2, 50–150 cells/field; 3, >150 cells/field). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31379843), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human PD-1 by ELISA Modulation of soluble co-signaling molecules in kidney-transplanted patients over time.The levels of the soluble co-signaling molecules CD30, CD40, CD137, CD40L, PD-1 and PD-L1 were assayed by ELISA in serum samples of healthy controls (n = 25) and kidney-transplanted patients (n = 59) obtained at different times: just before transplantation, and 15 days, 3 months and 1 year after transplantation. Data are shown as box-plots, in which the horizontal line within each box represents the median, the bottom and top of each box represent the 25th and 75th percentiles, the bars represent the 10th and 90th percentiles and circles indicate outliers. Unpaired and paired Wilcoxon tests were used to compare distributions between independent and dependent groups, respectively. * indicates statistically significant differences between healthy controls and kidney-transplanted patient samples, and † indicates statistically significant differences between patients samples obtained at different pre- and post-transplantation times. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25478957), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of PD‑1 in Human Tonsil. Formalin-fixed paraffin-embedded tissue sections of human tonsil were probed for PD1 mRNA (ACD RNAScope Probe, catalog #602021; Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-human PD1 polyclonal antibody (R&D Systems catalog # AF1086) at 1ug/mL with overnight incubation at 4 degrees Celsius followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to cell surface.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: PD-1
Programmed Death-1 (PD-1) is a type I transmembrane protein belonging to the CD28/CTLA-4 family of immunoreceptors that mediate signals for regulating immune responses (1). Members of the CD28/CTLA-4 family have been shown to either promote T cell activation (CD28 and ICOS) or down-regulate T cell activation (CTLA-4 and PD-1) (2). PD-1 is expressed on activated T cells, B cells, myeloid cells, and on a subset of thymocytes. In vitro, ligation of PD-1 inhibits TCR-mediated T-cell proliferation and production of IL-1, IL-4, IL-10, and IFN-gamma. In addition, PD-1 ligation also inhibits BCR mediated signaling. PD-1 deficient mice have a defect in peripheral tolerance and spontaneously develop autoimmune diseases (2, 3).
Two B7 family proteins, PD-L1 (also called B7-H1) and PD-L2 (also known as B7-DC), have been identified as PD-1 ligands. Unlike other B7 family proteins, both
PD‑L1 and PD-L2 are expressed in a wide variety of normal tissues including heart, placenta, and activated spleens (4). The wide expression of PD-L1 and PD-L2 and the inhibitor effects on PD-1 ligation indicate that PD-1 might be involved in the regulation of peripheral tolerance and may help prevent autoimmune diseases (2).
The human PD-1 gene encodes a 288 amino acid (aa) protein with a putative 20 aa signal peptide, a 148 aa extracellular region with one immunoglobulin-like V-type domain, a 24 aa transmembrane domain, and a 95 aa cytoplasmic region. The cytoplasmic tail contains two tyrosine residues that form the immuno-receptor
tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) that are important in mediating PD-1 signaling. Mouse and human PD-1 share approximately 60% aa sequence identity (4).
- Ishida, Y. et al. (1992) EMBO J. 11:3887.
- Nishimura, H. and T. Honjo (2001) Trends in Immunol. 22:265.
- Latchman, Y. et al. (2001) Nature Immun. 2:261.
- Carreno, B.M. and M. Collins (2002) Annu. Rev. Immunol. 20:29.
Product Datasheets
Citations for Human PD-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
65
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
PD-L1/PD-1 expression and tumor-infiltrating lymphocytes in conjunctival melanoma
Authors: Jinfeng Cao, Niels J. Brouwer, Kate E. Richards, Marina Marinkovic, Sjoerd van Duinen, Daan Hurkmans et al.
Oncotarget
-
Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles
Authors: P Minoo, I Zlobec, M Peterson, L Terracciano, A Lugli
International Journal of Oncology
-
TLR9 Mediated Tumor-Stroma Interactions in Human Papilloma Virus (HPV)-Positive Head and Neck Squamous Cell Carcinoma Up-Regulate PD-L1 and PD-L2
Authors: Paramita Baruah, Jessica Bullenkamp, Philip O. G. Wilson, Michael Lee, Juan Carlos Kaski, Ingrid E. Dumitriu
Frontiers in Immunology
-
Development and Fit-for-Purpose Validation of a Soluble Human Programmed Death-1 Protein Assay
Authors: Yan G. Ni, Xiling Yuan, John A. Newitt, Jon E. Peterson, Carol R. Gleason, Jonathan Haulenbeek et al.
The AAPS Journal
-
Gut germinal center regeneration and enhanced antiviral immunity by mesenchymal stem/stromal cells in SIV infection
Authors: Mariana G. Weber, Chara J. Walters-Laird, Amir Kol, Clarissa Santos Rocha, Lauren A. Hirao, Abigail Mende et al.
JCI Insight
-
Programmed Cell Death Ligand 1 Expression Is an Independent Prognostic Factor in Colorectal Cancer
Authors: TUMENJIN ENKHBAT, MASAAKI NISHI, CHIE TAKASU, KOZO YOSHIKAWA, HIGASHIJIMA JUN, TAKUYA TOKUNAGA et al.
Anticancer Research
-
The Impact of Indoleamine 2,3-dioxygenase (IDO) Expression on Stage III Gastric Cancer
Authors: MASAAKI NISHI, KOZO YOSHIKAWA, JUN HIGASHIJIMA, TAKUYA TOKUNAGA, HIDEYA KASHIHARA, CHIE TAKASU et al.
Anticancer Research
-
Nanoscale imaging of clinical specimens using conventional and rapid-expansion pathology
Authors: Octavian Bucur, Feifei Fu, Mike Calderon, Geetha H. Mylvaganam, Ngoc L. Ly, Jimmy Day et al.
Nature Protocols
-
Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease
Authors: Antonio Riva, Vishal Patel, Ayako Kurioka, Hannah C Jeffery, Gavin Wright, Sarah Tarff et al.
Gut
-
Rapid and Long-term Response of Pulmonary Pleomorphic Carcinoma to Nivolumab
Authors: Satoru Senoo, Takashi Ninomiya, Go Makimoto, Kazuya Nishii, Hirohisa Kano, Hiromi Watanabe et al.
Internal Medicine
-
Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection
Authors: Shohei Eto, Kozo Yoshikawa, Masaaki Nishi, Jun Higashijima, Takuya Tokunaga, Toshihiro Nakao et al.
Gastric Cancer
-
Programmed death-1–induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection
Authors: Elias A Said, Franck P Dupuy, Lydie Trautmann, Yuwei Zhang, Yu Shi, Mohamed El-Far et al.
Nature Medicine
-
Dimethyl Fumarate Targets MSK1, RSK1, 2 and IKK alpha / beta Kinases and Regulates NF-kappa B /p65 Activation in Psoriasis: A Demonstration of the Effect on Peripheral Blood Mononuclear Cells, Drawn from Two Patients with Severe Psoriasis Before and After Treatment with Dimethyl Fumarate
Authors: Borbala Gesser, Mads K Rasmussen, Lars Iversen
Psoriasis: Targets and Therapy
-
Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis
Authors: Madison Black, Ivraym B. Barsoum, Peter Truesdell, Tiziana Cotechini, Shannyn K. Macdonald-Goodfellow, Margaret Petroff et al.
Oncotarget
-
Comparison of Follicular Helper T-Cell Markers with the Expression of the Follicular Homing Marker CXCR5 in Peripheral T-Cell Lymphomas-A Reappraisal of Follicular Helper T-Cell Lymphomas
Authors: Krenács, L;Krenács, D;Borbényi, Z;Tóth, E;Nagy, A;Piukovics, K;Bagdi, E;
International journal of molecular sciences
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Modulation of immune checkpoint regulators in interferon ? induced urothelial carcinoma and activated T-lymphocyte cells by cytostatics
Authors: H�nze, J;Schulte-Herbr�ggen, J;Hofmann, R;Hegele, A;
Genes and immunity
Species: Human
Sample Types: Protein
Applications: Western Blot -
Exosomes in malignant pleural effusion from lung cancer patients impaired the cytotoxicity of double-negative T cells
Authors: J Wu, R Zhu, Z Wang, X Chen, T Xu, Y Liu, M Song, J Jiang, Q Ma, Z Chen, Y Liu, X Wang, M Zhang, M Huang, N Ji
Translational Oncology, 2022-10-14;27(0):101564.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer
Authors: AG Goubet, L Lordello, C Alves Cost, I Peguillet, M Gazzano, MD Mbogning-F, C Thelemaque, C Lebacle, C Thibault, F Audenet, G Pignot, G Gravis, C Helissey, L Campedel, M Roupret, E Xylinas, I Ouzaid, A Dubuisson, M Mazzenga, C Flament, P Ly, V Marty, N Signolle, A Sauvat, T Sbarrato, M Filahi, C Davin, G Haddad, J Bou Khalil, C Bleriot, FX Danlos, G Dunsmore, K Mulder, A Silvin, T Raoult, B Archambaud, S Belhechmi, I Gomperts B, N Cayet, M Moya-Nilge, A Mallet, R Daillere, E Rouleau, C Radulescu, Y Allory, J Fieschi, M Rouanne, F Ginhoux, G Le Teuff, L Derosa, A Marabelle, J VAN Dorp, N VAN Dijk, MS van der He, B Besse, F Andre, M Merad, G Kroemer, JY Scoazec, L Zitvogel, Y Loriot
Cancer Discovery, 2022-10-05;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Ex vivo-expanded human CD19+TIM-1+ regulatory B cells suppress immune responses in vivo and are dependent upon the TIM-1/STAT3 axis
Authors: S Shankar, J Stolp, SC Juvet, J Beckett, PS Macklin, F Issa, J Hester, KJ Wood
Nature Communications, 2022-06-03;13(1):3121.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Additive Intralesional Interleukin-2 Improves Progression-Free Survival in a Distinct Subgroup of Melanoma Patients with Prior Progression under Immunotherapy
Authors: D Rafei-Sham, S Lehr, M Behrens, F Meiss
Cancers, 2022-01-21;14(3):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The role of the immunoescape in colorectal cancer liver metastasis
Authors: C Takasu, S Yamashita, Y Morine, K Yoshikawa, T Tokunaga, M Nishi, H Kashihara, T Yoshimoto, M Shimada
PLoS ONE, 2021-11-19;16(11):e0259940.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
CD4+ T Cells Are Dispensable for Induction of Broad Heterologous HIV Neutralizing Antibodies in Rhesus Macaques
Authors: Sarkar S, Spencer DA, Barnette P et al.
Frontiers in Immunology
-
Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling
Authors: C Glorieux, X Xia, YQ He, Y Hu, K Cremer, A Robert, J Liu, F Wang, J Ling, PJ Chiao, P Huang
Redox Biology, 2020-11-03;38(0):101780.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Impact of sidedness of colorectal cancer on tumor immunity
Authors: C Takasu, M Nishi, K Yoshikawa, T Tokunaga, H Kashihara, T Yoshimoto, M Shimada
PLoS ONE, 2020-10-12;15(10):e0240408.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The innate immune effector ISG12a promotes cancer immunity by suppressing the canonical Wnt/&beta-catenin signaling pathway
Authors: R Deng, C Zuo, Y Li, B Xue, Z Xun, Y Guo, X Wang, Y Xu, R Tian, S Chen, Q Liu, J Chen, J Wang, X Huang, H Li, M Guo, X Wang, M Yang, Z Wu, J Wang, J Ma, J Hu, G Li, S Tang, Z Tu, H Ji, H Zhu
Cell. Mol. Immunol., 2020-09-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Concordance of PD-1 and PD-L1 (B7-H1) in paired primary and metastatic clear cell renal cell carcinoma
Authors: JE Eckel-Pass, TH Ho, DJ Serie, JC Cheville, R Houston Th, BA Costello, H Dong, ED Kwon, BC Leibovich, AS Parker
Cancer Med, 2019-12-12;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Mechanisms utilized by feline adipose-derived mesenchymal stem cells to inhibit T lymphocyte proliferation
Authors: N Taechangam, SS Iyer, NJ Walker, B Arzi, DL Borjesson
Stem Cell Res Ther, 2019-06-25;10(1):188.
Species: Feline
Sample Types: Whole Cells
Applications: Flow Cytometry -
m6A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade
Authors: S Yang, J Wei, YH Cui, G Park, P Shah, Y Deng, AE Aplin, Z Lu, S Hwang, C He, YY He
Nat Commun, 2019-06-25;10(1):2782.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Low levels of SIV-specific CD8+ T cells in germinal centers characterizes acute SIV infection
Authors: S Li, JM Folkvord, KJ Kovacs, RK Wagstaff, G Mwakalundw, AK Rendahl, EG Rakasz, E Connick, PJ Skinner
PLoS Pathog., 2019-03-21;15(3):e1007311.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
Tumor-derived exosomal HMGB1 promotes esophageal squamous cell carcinoma progression through inducing PD1+ TAM expansion
Authors: B Li, TN Song, FR Wang, C Yin, Z Li, JP Lin, YQ Meng, HM Feng, T Jing
Oncogenesis, 2019-02-22;8(3):17.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Analysis of expression of the PD-1/PD-L1 immune checkpoint system and its prognostic impact in gastroenteropancreatic neuroendocrine tumors
Authors: M Sampedro-N, A Serrano-So, M Adrados, JM Cameselle-, C Blanco-Car, JM Cabezas-Ag, R Martínez-H, E Martín-Pér, JL Muñoz de N, JÁ Díaz, R García-Cen, J Caneiro-Gó, I Abdulkader, R González-A, M Marazuela
Sci Rep, 2018-12-13;8(1):17812.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression
Authors: F Wang, B Li, Y Wei, Y Zhao, L Wang, P Zhang, J Yang, W He, H Chen, Z Jiao, Y Li
Oncogenesis, 2018-05-25;7(5):41.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: Functional Assay, IHC -
Altered Ratio of T Follicular Helper Cells to T Follicular Regulatory Cells Correlates with Autoreactive Antibody Response in Simian Immunodeficiency Virus-Infected Rhesus Macaques
Authors: W Fan, AJ Demers, Y Wan, Q Li
J. Immunol., 2018-04-02;0(0):.
Species: Primate
Sample Types: Whole Cells
Applications: Flow Cytometry -
Associations of Simian Immunodeficiency Virus (SIV)-Specific Follicular CD8+T Cells with Other Follicular T Cells Suggest Complex Contributions to SIV Viremia Control
Authors: MA Rahman, KM McKinnon, TS Karpova, DA Ball, DJ Venzon, W Fan, G Kang, Q Li, M Robert-Gur
J. Immunol., 2018-03-05;0(0):.
Species: Primate
Sample Types: Whole Tissue
Applications: IHC -
Blockade of Tumor-Expressed PD-1 promotes lung cancer growth
Authors: S Du, N McCall, K Park, Q Guan, P Fontina, A Ertel, T Zhan, AP Dicker, B Lu
Oncoimmunology, 2018-01-29;7(4):e1408747.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Quantitative Multiplexed Imaging Analysis Reveals a Strong Association between Immunogen-Specific B Cell Responses and Tonsillar Germinal Center Immune Dynamics in Children after Influenza Vaccination
Authors: D Amodio, N Cotugno, G Macchiarul, S Rocca, Y Dimopoulos, MR Castrucci, R De Vito, FM Tucci, AB McDermott, S Narpala, P Rossi, RA Koup, P Palma, C Petrovas
J. Immunol., 2017-12-13;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers
Authors: Y Liu, Y Cheng, Y Xu, Z Wang, X Du, C Li, J Peng, L Gao, X Liang, C Ma
Oncogene, 2017-07-10;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay, Neutralization -
Immune Cell Dynamics in Rhesus Macaques Infected with a Brazilian Strain of Zika Virus
Authors: ELV Silveira, KA Rogers, S Gumber, P Amancha, P Xiao, SM Woollard, SN Byrareddy, MM Teixeira, F Villinger
J. Immunol., 2017-06-30;0(0):.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
Particulate Array of Well-Ordered HIV Clade C Env Trimers Elicits Neutralizing Antibodies that Display a Unique V2 Cap Approach
Authors: P Martinez-M, K Tran, J Guenaga, G Lindgren, M Àdori, Y Feng, GE Phad, N Vázquez Be, S Bale, J Ingale, V Dubrovskay, S O'Dell, L Pramanik, M Spångberg, M Corcoran, K Loré, JR Mascola, RT Wyatt, GB Karlsson H
Immunity, 2017-05-16;46(5):804-817.e7.
Species: Primate
Sample Types: Whole Tissue
Applications: IHC -
PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome
Authors: Joseph M Obeid
Oncoimmunology, 2016-09-20;5(11):e1235107.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma
Authors: PT Nghiem, S Bhatia, EJ Lipson, RR Kudchadkar, NJ Miller, L Annamalai, S Berry, EK Chartash, A Daud, SP Fling, PA Friedlande, HM Kluger, HE Kohrt, L Lundgren, K Margolin, A Mitchell, T Olencki, DM Pardoll, SA Reddy, EM Shantha, WH Sharfman, E Sharon, She
N Engl J Med, 2016-04-19;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
Vaccine Induction of Lymph Node-Resident Simian Immunodeficiency Virus Env-Specific T Follicular Helper Cells in Rhesus Macaques.
Authors: Vargas-Inchaustegui D, Demers A, Shaw J, Kang G, Ball D, Tuero I, Musich T, Mohanram V, Demberg T, Karpova T, Li Q, Robert-Guroff M
J Immunol, 2016-01-15;196(4):1700-10.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion.
Authors: Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, Nie W, Reszka-Blanco N, Wang F, Su L
J Clin Invest, 2015-08-24;125(9):3692-703.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Soluble co-signaling molecules predict long-term graft outcome in kidney-transplanted patients.
Authors: Melendreras S, Martinez-Camblor P, Menendez A, Bravo-Mendoza C, Gonzalez-Vidal A, Coto E, Diaz-Corte C, Ruiz-Ortega M, Lopez-Larrea C, Suarez-Alvarez B
PLoS ONE, 2014-12-05;9(12):e113396.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Early lymphoid responses and germinal center formation correlate with lower viral load set points and better prognosis of simian immunodeficiency virus infection.
Authors: Hong J, Amancha P, Rogers K, Courtney C, Havenar-Daughton C, Crotty S, Ansari A, Villinger F
J Immunol, 2014-06-06;193(2):797-806.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
CD4 T follicular helper cell dynamics during SIV infection.
J. Clin. Invest., 2012-08-27;122(9):3281-94.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC-P -
Modulation of T-cell activation by malignant melanoma initiating cells.
Authors: Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, Zhou J, Hodi FS, Spagnoli GC, Murphy GF, Frank MH
Cancer Res., 2010-01-12;70(2):697-708.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques.
Authors: Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT
J. Immunol., 2008-05-15;180(10):6798-807.
Species: Primate - Macaca mulatta (Rhesus Macaque), Primate - Cercocebus atys (Sooty Mangabey)
Sample Types: Whole Tissue
Applications: IHC -
Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection.
Authors: Onlamoon N, Rogers K, Mayne AE, Pattanapanyasat K, Mori K, Villinger F, Ansari AA
Immunology, 2008-02-05;124(2):277-93.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Neutralization -
Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis.
Authors: Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ
J. Immunol., 2006-12-15;177(12):8844-50.
Species: Human
Sample Types: Serum
Applications: ELISA Development -
Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients
Authors: Marie-Nicole Theodoraki, Saigopalakrishna S. Yerneni, Thomas K. Hoffmann, William E. Gooding, Theresa L. Whiteside
Clinical Cancer Research
-
A B cell follicle sanctuary permits persistent productive SIV infection in elite controllers.
Authors: Fukazawa Y, Lum R, Okoye AA et al.
Nat Med
-
Multiple Immunostainings with Different Epitope Retrievals—The FOLGAS Protocol
Authors: Anna von Schoenfeld, Peter Bronsert, Michael Poc, Andrew Fuller, Andrew Filby, Stefan Kraft et al.
International Journal of Molecular Sciences
-
Differential Expression of Programmed Death-1 (PD-1) in Sézary Syndrome and Mycosis Fungoides
Authors: Fatma Çetinözman, Patty M. Jansen, Maarten H. Vermeer, Rein Willemze
Archives of Dermatology
-
TIA-1 Cytotoxic Granule-Associated RNA Binding Protein Improves the Prognostic Performance of CD8 in Mismatch Repair-Proficient Colorectal Cancer
Authors: Inti Zlobec, Eva Karamitopoulou, Luigi Terracciano, Salvatore Piscuoglio, Giandomenica Iezzi, Manuele Giuseppe Muraro et al.
PLoS ONE
-
The Intrahepatic Expression and Distribution of BTLA and its Ligand HVEM in patients with HBV-related acute-on-chronic liver failure
Authors: Huan Xu, Dayan Cao, Guoning Guo, Zhihua Ruan, Yuzhang Wu, Yongwen Chen
Diagnostic Pathology
-
Cancer cell intrinsic TIM-3 induces glioblastoma progression
Authors: Qing Guo, Shuai Shen, Gefei Guan, Chen Zhu, Cunyi Zou, Jingyuan Cao et al.
iScience
-
CD4+ T Cells Are Dispensable for Induction of Broad Heterologous HIV Neutralizing Antibodies in Rhesus Macaques
Authors: Sarkar S, Spencer DA, Barnette P et al.
Frontiers in Immunology
-
The Possible Role of PD-1 Protein in Ganoderma lucidum-Mediated Immunomodulation and Cancer Treatment
Authors: Gan Wang, Le Wang, Jianlong Zhou, Xiaoxin Xu
Integr Cancer Ther
-
Cellular and soluble immune checkpoint signaling forms PD-L1 and PD-1 in renal tumor tissue and in blood
Authors: Corinna U. Keber, Marcus Derigs, Carolin Schultz, Moritz Wegner, Susanne Lingelbach, Viktoria Wischmann et al.
Cancer Immunology, Immunotherapy
-
PD-1 expression and clinical PD-1 blockade in B-cell lymphomas
Authors: Zijun Y. Xu-Monette, Jianfeng Zhou, Ken H. Young
Blood
-
Anti‐PD‐L1/TGF‐ beta R fusion protein (SHR‐1701) overcomes disrupted lymphocyte recovery‐induced resistance to PD‐1/PD‐L1 inhibitors in lung cancer
Authors: Bo Cheng, Kaikai Ding, Pengxiang Chen, Jianxiong Ji, Tao Luo, Xiaofan Guo et al.
Cancer Communications
-
Anti-PD1 ‘SHR-1210ʹ aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement
Authors: William J.J. Finlay, James E. Coleman, Jonathan S. Edwards, Kevin S. Johnson
mAbs
-
Tumor-endogenous PD-1 promotes cell proliferation and predicts poor survival in non-small cell lung cancer
Authors: Fanyi Gan, Chuanfen Zhang, Liang Xia, Senyi Deng
Translational Cancer Research
-
Targeting the PD1/PD-L1 axis in melanoma: Biological rationale, clinical challenges and opportunities
Authors: Barbara Merelli, Daniela Massi, Laura Cattaneo, Mario Mandalà
Critical Reviews in Oncology/Hematology
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human PD-1 Antibody
Average Rating: 4.6 (Based on 7 Reviews)
Have you used Human PD-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
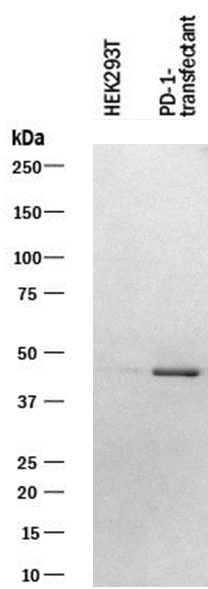
Letivirus carrying human PD-1 was used to infect 293T cells. The membrane was exposed to a concentration of 1 µg/mL Human PD‑1 Monoclonal Antibody. PD‑1 band was identified at approximately 45 kDa.
293T cells were infected by lentivirus overexpression of control or PD-1 for 24 and 48 h. Total cell lysates were subjected to western blot. PVDF membrane were probed with 1 um/ml Human PD-1 Antibody (AF1086). A specific band was detected for PD-1 at approximately 43 kDa. This experiment was conducted under reducing conditions
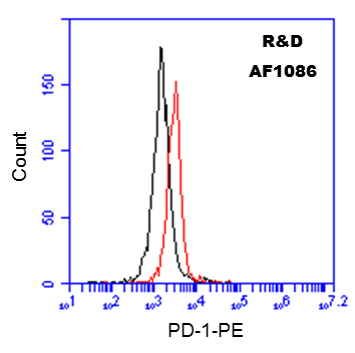
10^6 Human PBMCs were probed with 0.5 ug of Goat anti-Human PD-1 antibody (red), or Goat Isotype Control IgG (black), followed by PE-conjugated anti-Goat secondary antibody.