Human Neuropilin-1 Antibody Summary
Phe22-Lys644
Accession # NP_001019799
Applications
(Catalog # 3870‑N1) coated at 0.75 µg/mL (100 µL/well). At 5 µg/mL, this antibody will block >90% of the binding.
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
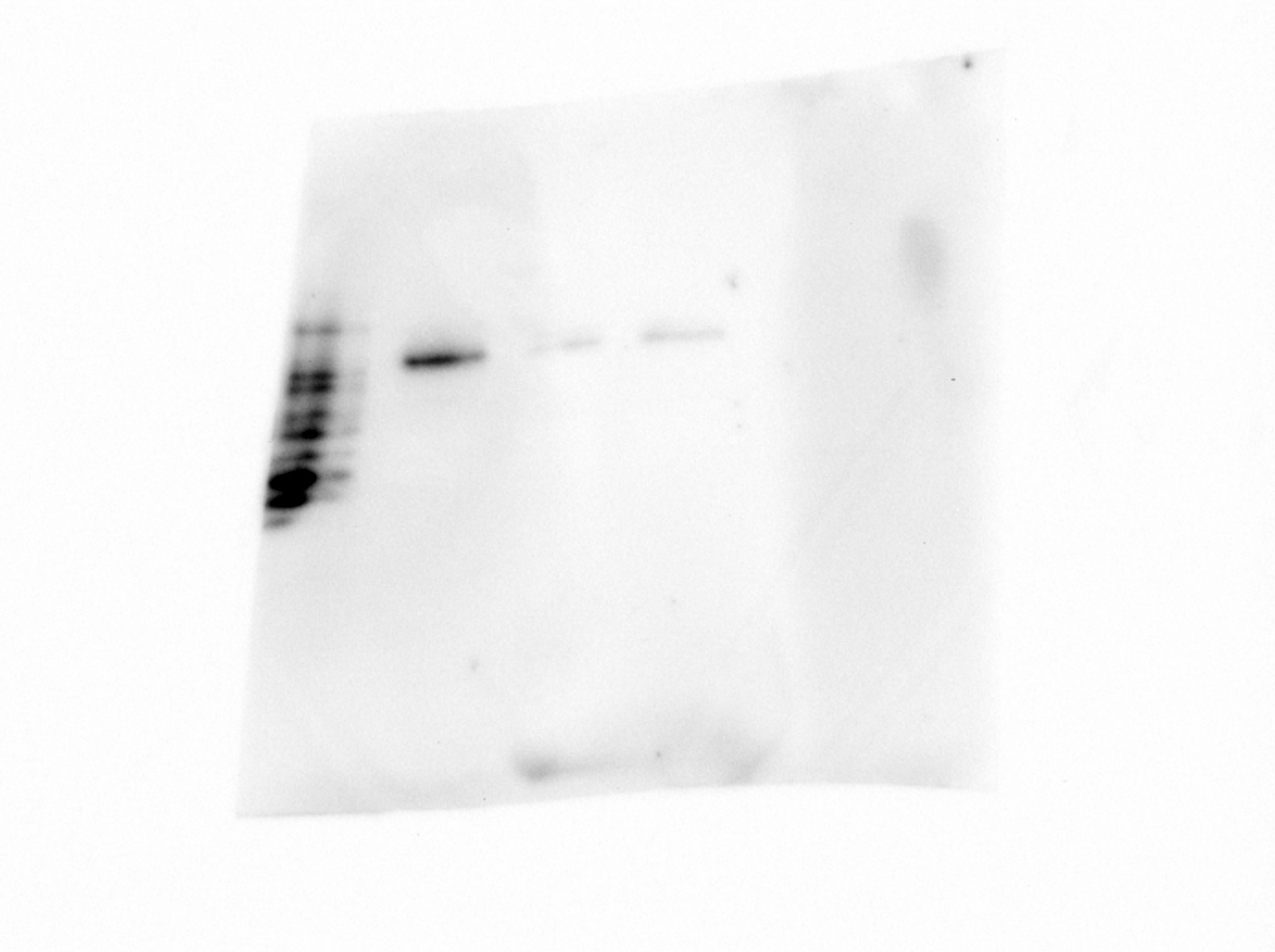
Detection of Human Neuropilin‑1 by Western Blot. Western blot shows lysates of MDA‑MB‑231 human breast cancer cell line and human placenta tissue. PVDF membrane was probed with 1 µg/mL of Sheep Anti-Human Neuropilin‑1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3870) followed by HRP-conjugated Anti-Sheep IgG Secondary Antibody (Catalog # HAF016). A specific band was detected for Neuropilin‑1 at approximately 130 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of Neuropilin‑1 in HUVEC Human Cells by Flow Cytometry. HUVEC human umbilical vein endothelial cells were stained with Sheep Anti-Human Neuropilin-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3870, filled histogram) or control antibody (Catalog # 5-001-A, open histogram), followed by NorthernLights™ 637-conjugated Anti-Sheep IgG Secondary Antibody (Catalog # NL011).
 View Larger
View Larger
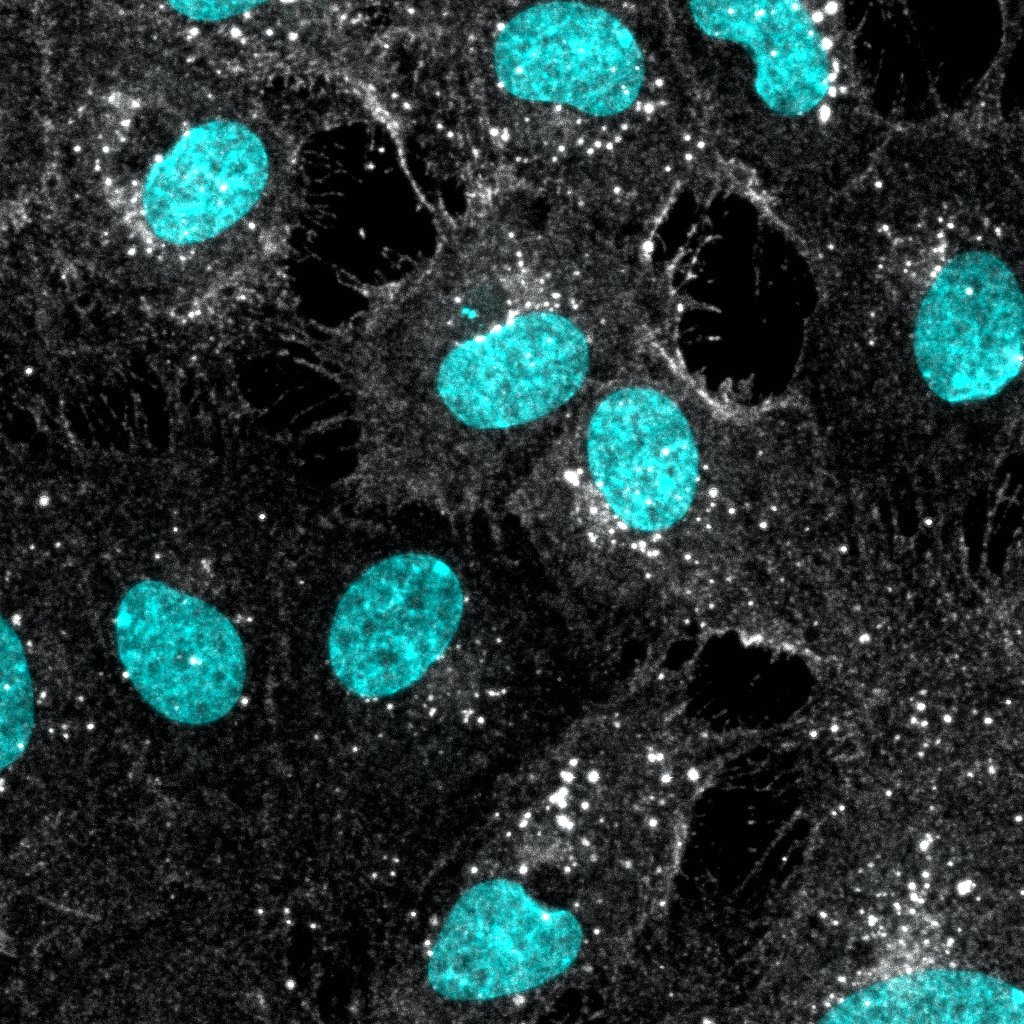
Neuropilin‑1 in PC-3 and HLDM-2 Human Cell Lines. Neuropilin-1 was detected in immersion fixed PC-3 human prostate cancer cell line (positive staining) and HLDM-2 human Hodgkin's lymphoma cell line (negative staining) using Sheep Anti-Human Neuropilin-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3870) at 5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Sheep IgG Secondary Antibody (red; Catalog # NL010) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Neuropilin-1 in Human Pancreatic Cancer Tissue. Neuropilin-1 was detected in immersion fixed paraffin-embedded sections of human pancreatic cancer tissue using Sheep Anti-Human Neuropilin-1 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF3870) at 10 µg/mL overnight at 4 °C. Before incubation with the primary antibody tissue was subjected to heat-induced epitope retrieval using Antigen Retrieval Reagent-Basic (Catalog # CTS013). Tissue was stained using the Anti-Sheep HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS019) and counterstained with hematoxylin (blue). Specific labeling was localized to the cytoplasm and plasma membrane of cancer cells. View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
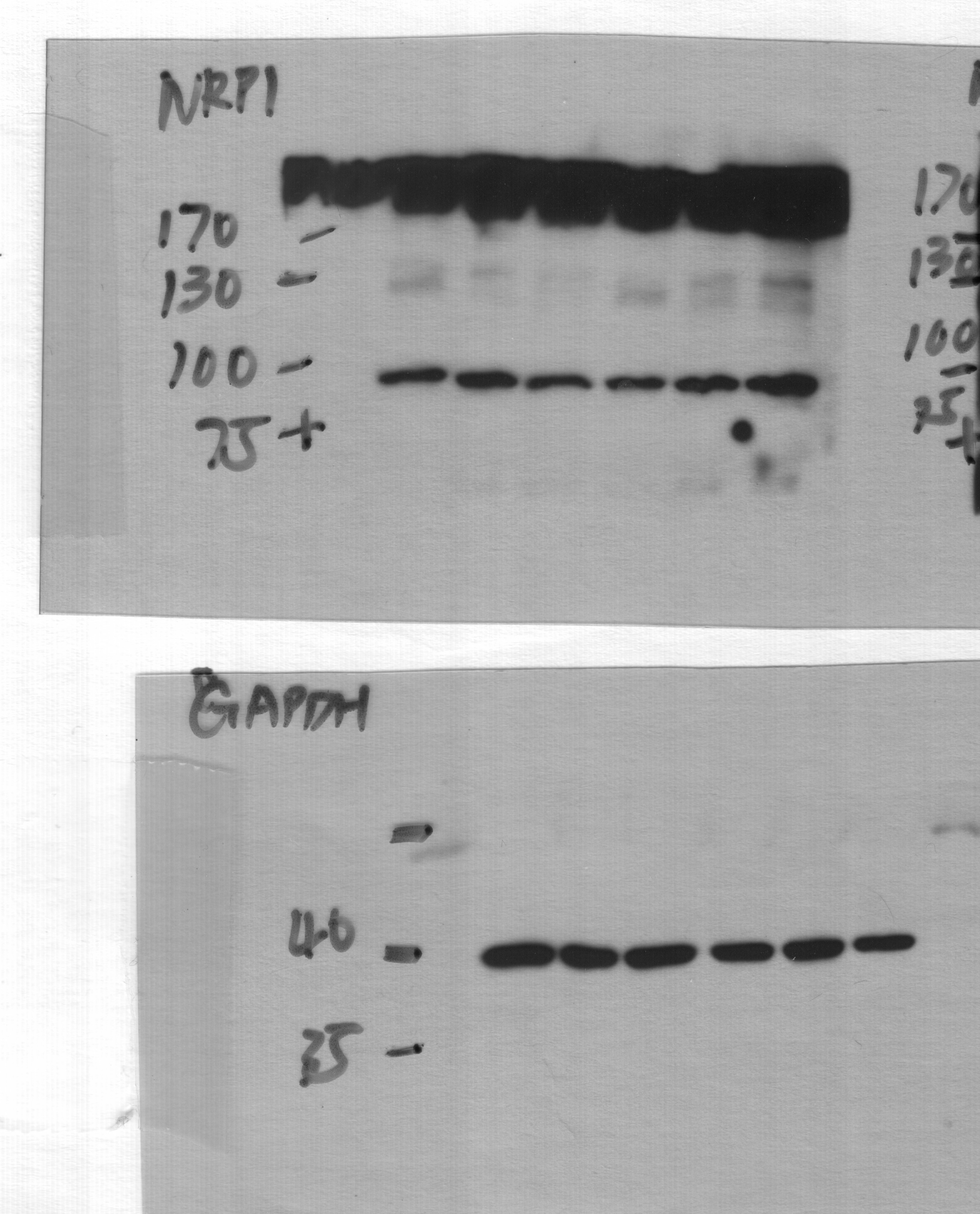
Detection of Human Neuropilin-1 by Knockdown Validated NRP1-dependent EGFR/ERK signalling pathways are activated by EBV and are also associated with the promotion of EBV infection.(a,b) EBV infection activated EGFR/AKT and EGFR/ERK pathways. Serum-starved HNE1 cells were infected with EBV (a) or purified EBV (b) at an MOI of 5 × 103 for the indicated times. (c) EBV-activated EGFR/AKT or EGFR/ERK pathways were partially mediated by NRP1. After transfected with the indicated siRNA duplexes for 48 h, HNE1 cells were serum-starved and infected with EBV at an MOI of 5 × 103 for 1 h, followed by western blotting. For a–c, the intensity of the bands, determined with the ImageJ software, was shown as indicated. alpha -Tubulin, internal control. (d) Schematic diagram of RTKs (EGFR and VEGFR2)/NRP1/Ras/ERK and RTKs/NRP1/PI3K/AKT signalling cascade inhibitors targeting the indicated members of the pathways are shown. (e) EGFR/Ras/ERK signalling pathway was required for EBV infection. EGF-treated HNE1 and NPEC1-Bmi1 cells pre-incubated with Gefitinib (20 μM), Sorafenib (HNE1, 20 μM; NPEC1-Bmi1, 5 μM), U0126 (50 μM) and LY294002 (50 μM) for 30 min were infected with EBV for 2 h, and then cultured in the fresh medium for 48 h until FACS analysis for the infection efficiency. (f,g) Downregulation of EGFR and c-Met impaired EBV infection. HNE1 cells were transfected with siRNA duplexes targeting EGFR or c-Met for 48 h, followed by real-time PCR analysis for the expressions of EGFR and c-Met (f) or FACS analysis for EBV infection efficiency (g). (h) HRas partially rescued the inhibitory effect of Gefitinib on EBV infection. HNE1 cells transfected with pMSCV-HRAS-V12 plasmid for 24 h were pre-incubated with 20 μM Gefitinib for 30 min, followed by exposure to EBV. (i) HRas failed to rescue the suppressive effect of silencing NRP1 on EBV infection. After transfected with siNRP1 or siControl for 36 h, HNE1 cells were reseeded, and then transfected with the plasmid for HRAS-V12 or the empty vector for 24 h, followed by exposure to EBV. The infected cells were analysed by FACS, with controls set to 100%. For (e–i), data represent three times independent experiments. Graphs show mean±s.e.m. ***P<0.001; **P<0.01; *P<0.05; Student’s t-test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25670642), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Neuropilin-1 by Knockdown Validated NRP1 enhances EBV infection, while NRP2 suppresses EBV infection.(a,b) Downregulation of NRP1 impaired, whereas knockdown of NRP2 promoted EBV infection. HNE1 cells were transfected with siRNA duplexes targeting NRP1 or NRP2 for 48 h, followed by NRP expression analysis by real-time PCR (a) or analysis for the efficiency of EBV infection (b); n=3. (c) EBV infection was blocked by soluble NRP1ABC, but enhanced by antibody against NRP2. For NRP1ABC protein-blocking experiment, HNE1 cells were infected with EBV, which was pre-incubated with purified NRP1ABC for 1 h. For antibody against NRP2-blocking experiment, HNE1 cells were pre-incubated with an anti-NRP2 antibody (100 μg ml−1) or goat IgG (control) at 4 °C for 1 h and then were exposed to EBV at an MOI of 5 × 103 for 3 h at 4 °C. (d,e) Overexpression of NRP1 enhanced EBV infection, while NRP2 suppressed EBV infection. HNE1 cells were transiently transfected with the expression plasmid for NRP1, NRP2 or the empty vector (pMSCV) for 24 h, followed by analysis for the expression of NRP1 and NRP2 by western blotting (d) or were exposed to EBV (e). (f,g) EGF upregulated NRP1 expression and enhanced EBV infection. HNE1 cells cultured with 10 ng ml−1 EGF for 24 h were analysed for the expression of NRP1 and NRP2 by western blotting (f) or were exposed to EBV (g). (h,i) EGF-enhanced EBV infection was partially dependent on NRP1. After transfected with siRNA against NRP1 for 48 h, EGF-treated HNE1 and NPEC-Bmi1 cells maintained in KSF medium supplemented with EGF were analysed for NRP1 expression by western blotting (h) or were exposed to EBV (i). For (b), (c), (e), (g) and (i), HNE1 or NPEC1-Bmi1 cells were exposed to EBV at an MOI of 2.5 × 103 for 2 h at 37 °C, unless otherwise indicated. The percentage of GFP-positive infected cells was analysed by FACS 48 h post infection, with controls (empty vector-transfected cells or vehicle treated-cells) set to 100%. Data represent three to five independent experiments. Values in all graphs are means±s.e.m. ***P<0.001; **P<0.01; *P<0.05; Student’s t-test. For (d), (f) and (h), GAPDH served as an internal control. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25670642), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Neuropilin-1
Neuropilin-1 (Npn-1, previously neuropilin; also CD304/BDCA4) is a 130-140 kDa type I transmembrane (TM) glycoprotein that regulates axon guidance and angiogenesis (1-4). The full-length 923 amino acid (aa) human Npn-1 contains a 623 aa extracellular domain (ECD) that shows 92-95% aa identity with mouse, rat, bovine, and canine Npn-1 (3, 4). The ECD contains two N-terminal CUB domains (termed a1a2), two domains with homology to coagulation factors V and VIII (b1b2) and a MAM (meprin) domain (c). C-terminally divergent splice variants with 704, 644, 609, and 551 aa lack the MAM and TM domains and are demonstrated or presumed to be soluble antagonists (1, 5-7). A 906 aa form lacks a TM segment, but secretion has not been found (8). The sema domains of Class III secreted semaphorins such as Sema3A bind Npn-1 a1a2 (9). Heparin, the heparin-binding forms of VEGF (VEGF165, VEGF-B, and VEGF-E), PlGF (PlGF‑2), and the C-terminus of Sema3 bind the b1b2 region (9, 10). Npn-1 and Npn-2 share 48% aa identity within the ECD and can form homo- and hetero-oligomers via interaction of their MAM domains (1). Neuropilins show partially overlapping expression in neuronal and endothelial cells during development (1, 2). Both neuropilins act as co-receptors with plexins, mainly plexin A3 and A4, to bind class III semaphorins that mediate axon repulsion (11). However, only Npn-1 binds Sema3A, and only Npn-2 binds Sema3F (1). Both are co-receptors with VEGF R2 (also called KDR or Flk-1) for VEGF165 binding (1). Sema3A signaling can be blocked by VEGF165, which has higher affinity for Npn-1 (12). Npn-1 is preferentially expressed in arteries during development or those undergoing remodeling (1, 2). Npn-1 is also expressed on dendritic cells and mediates DC-induced T cell proliferation (13).
- Bielenberg, D.R. et al. (2006) Exp. Cell Res. 312:584.
- Gu, C. et al. (2003) Dev. Cell 5:45.
- He, Z. and M. Tessier-Lavigne (1997) Cell 90:739.
- Soker, S. et al. (1998) Cell 92:735.
- Gagnon, M.L. et al. (2000) Proc. Natl. Acad. Sci. USA 97:2573.
- Cackowski, F.C. et al. (2004) Genomics 84:82.
- Rossignol, M. et al. (2000) Genomics 70:211.
- Tao, Q. et al. (2003) Angiogenesis 6:39.
- Gu, C. et al. (2002) J. Biol. Chem. 277:18069.
- Mamluk, R. et al. (2002) J. Biol. Chem. 277:24818.
- Yaron, A. et al. (2005) Neuron 45:513.
- Narazaki, M. and G. Tosato (2006) Blood 107:3892.
- Tordjman, R. et al. (2002) Nat. Immunol. 3477.
Product Datasheets
Product Specific Notices
This product or the use of this product is covered by U.S. Patents owned by The Regents of the University of California. This product is for research use only and is not to be used for commercial purposes. Use of this product to produce products for sale or for diagnostic, therapeutic or drug discovery purposes is prohibited. In order to obtain a license to use this product for such purposes, contact The Regents of the University of California.U.S. Patent # 6,054,293, 6,623,738, and other U.S. and international patents pending.
Citations for Human Neuropilin-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
22
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling
Authors: Vedanta Mehta, Laura Fields, Ian M. Evans, Maiko Yamaji, Caroline Pellet-Many, Timothy Jones et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy
Authors: Lissenya B. Argueta, Lauretta A. Lacko, Yaron Bram, Takuya Tada, Lucia Carrau, André Figueiredo Rendeiro et al.
iScience
-
SARS-CoV-2 infection induces beta cell transdifferentiation
Authors: Tang X, Uhl S, Zhang T et al.
Cell Metabolism
-
Rhodocetin-alpha beta –induced Neuropilin-1–cMet Association Triggers Restructuring of Matrix Contacts in Endothelial Cells
Authors: Stephan Niland, Bartosz Ditkowski, Désirée Parrandier, Lise Roth, Hellmut Augustin, Johannes A. Eble
Arteriosclerosis, Thrombosis, and Vascular Biology
-
alpha2,6 Sialylation mediated by ST6GAL1 promotes glioblastoma growth
Authors: S Gc, K Tuy, L Rickenback, R Jones, A Chakrabort, CR Miller, EA Beierle, VS Hanumanthu, AN Tran, JA Mobley, SL Bellis, AB Hjelmeland
JCI Insight, 2022-11-08;7(21):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Neuropilin 1 and its inhibitory ligand mini-tryptophanyl-tRNA synthetase inversely regulate VE-cadherin turnover and vascular permeability
Authors: N Gioelli, LJ Neilson, N Wei, G Villari, W Chen, B Kuhle, M Ehling, F Maione, S Willox, S Brundu, D Avanzato, G Koulouras, M Mazzone, E Giraudo, XL Yang, D Valdembri, S Zanivan, G Serini
Nature Communications, 2022-07-20;13(1):4188.
Species: Human
Sample Types: Whole Cells
Applications: ELISA Capture -
Differential plasmacytoid dendritic cell phenotype and type I Interferon response in asymptomatic and severe COVID-19 infection
Authors: M Severa, RA Diotti, MP Etna, F Rizzo, S Fiore, D Ricci, M Iannetta, A Sinigaglia, A Lodi, N Mancini, E Criscuolo, M Clementi, M Andreoni, S Balducci, L Barzon, P Stefanelli, N Clementi, EM Coccia
PloS Pathogens, 2021-09-02;17(9):e1009878.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
SARS-CoV-2 Infects Syncytiotrophoblast and Activates Inflammatory Responses in the Placenta
Authors: LB Argueta, LA Lacko, Y Bram, T Tada, L Carrau, T Zhang, S Uhl, BC Lubor, V Chandar, C Gil, W Zhang, B Dodson, J Bastiaans, M Prabhu, CM Salvatore, YJ Yang, RN Baergen, BR tenOever, NR Landau, S Chen, RE Schwartz, H Stuhlmann
bioRxiv : the preprint server for biology, 2021-06-17;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Semaphorin 3F signaling actively retains neutrophils at sites of inflammation
Authors: T Plant, S Eamsamarng, MA Sanchez-Ga, L Reyes, SA Renshaw, P Coelho, AS Mirchandan, JM Morgan, FE Ellett, T Morrison, D Humphries, ER Watts, F Murphy, XL Raffo-Irao, A Zhang, JL Cash, C Loynes, PM Elks, F Van Eeden, LM Carlin, AJW Furley, MKB Whyte, SR Walmsley
J. Clin. Invest., 2020-06-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Atypical chemokine receptor ACKR3/CXCR7 controls postnatal vasculogenesis and arterial specification by mesenchymal stem cells via Notch signaling
Authors: ST Wei, YC Huang, ML Hsieh, YJ Lin, WC Shyu, HC Chen, CH Hsieh
Cell Death Dis, 2020-05-04;11(5):307.
Species: Human
Sample Types: Cell Culture Supernates
Applications: Western Blot -
iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer
Authors: N Ding, Z Zou, H Sha, S Su, H Qian, F Meng, F Chen, S Du, S Zhou, H Chen, L Zhang, J Yang, J Wei, B Liu
Nat Commun, 2019-03-22;10(1):1336.
Species: Human
Sample Types: Spheroids
Applications: Neutralization -
Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output
Authors: SL Giandomeni, SB Mierau, GM Gibbons, LMD Wenger, L Masullo, T Sit, M Sutcliffe, J Boulanger, M Tripodi, E Derivery, O Paulsen, A Lakatos, MA Lancaster
Nat. Neurosci., 2019-03-18;22(4):669-679.
Species: Human
Sample Types: Organoid
Applications: IHC -
Neuropilin-1 mediates Neutrophil Elastase uptake and cross-presentation in breast cancer cells
Authors: C Kerros, SC Tripathi, D Zha, JM Mehrens, A Sergeeva, AV Philips, N Qiao, HL Peters, H Katayama, P Sukhumalch, KE Ruisaard, AA Perakis, LS St John, S Lu, EA Mittendorf, K Clise-Dwye, AC Herrmann, G Alatrash, C Toniatti, SM Hanash, Q Ma, JJ Molldrem
J. Biol. Chem., 2017-05-03;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Conformational remodeling of the fibronectin matrix selectively regulates VEGF signaling.
Authors: Ambesi A, McKeown-Longo P
J Cell Sci, 2014-06-30;127(0):3805-16.
Species: Human
Sample Types: Whole Cells
Applications: Western Blot -
Instruction of circulating endothelial progenitors in vitro towards specialized blood-brain barrier and arterial phenotypes.
Authors: Boyer-Di Ponio J, El-Ayoubi F, Glacial F, Ganeshamoorthy K, Driancourt C, Godet M, Perriere N, Guillevic O, Couraud P, Uzan G
PLoS ONE, 2014-01-02;9(1):e84179.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas.
Authors: Pellet-Many C, Frankel P, Evans IM, Herzog B, Junemann-Ramirez M, Zachary IC
Biochem. J., 2011-05-01;435(3):609-18.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis.
Authors: Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM
J. Biol. Chem., 2009-10-16;284(49):33966-81.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Human Semaphorin 3 Variants Link Melanocortin Circuit Development and Energy Balance
Authors: Agatha A. van der Klaauw, Sophie Croizier, Edson Mendes de Oliveira, Lukas K.J. Stadler, Soyoung Park, Youxin Kong et al.
Cell
-
VEGF pathway-targeting drugs induce evasive adaptation by activation of neuropilin-1/cMet in colon cancer cells
Authors: Chisato Tomida, Naoko Yamagishi, Hikaru Nagano, Takayuki Uchida, Ayako Ohno, Katsuya Hirasaka et al.
International Journal of Oncology
-
Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells.
Authors: Wang, Hong-Bo, Zhang, Hua, Zhang, Jing-Pin, Li, Yan, Zhao, Bo, Feng, Guo-Kai, Du, Yong, Xiong, Dan, Zhong, Qian, Liu, Wan-Li, Du, Huamao, Li, Man-Zhi, Huang, Wen-Lin, Tsao, Sai Wah, Hutt-Fletcher, Lindsey, Zeng, Yi-Xin, Kieff, Elliott, Zeng, Mu-Sheng
Nat Commun, 2015-02-11;6(0):6240.
-
Neuropilin 1 and Neuropilin 2 gene invalidation or pharmacological inhibition reveals their relevance for the treatment of metastatic renal cell carcinoma
Authors: Aurore Dumond, Etienne Brachet, Jérôme Durivault, Valérie Vial, Anna K. Puszko, Yves Lepelletier et al.
Journal of Experimental & Clinical Cancer Research
-
Neuropilin-1 signaling through p130Cas tyrosine phosphorylation is essential for growth factor-dependent migration of glioma and endothelial cells.
Authors: Evans IM, Yamaji M, Britton G, Pellet-Many C, Lockie C, Zachary IC, Frankel P
Mol. Cell. Biol., 2011-01-18;31(6):1174-85.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human Neuropilin-1 Antibody
Average Rating: 3.8 (Based on 5 Reviews)
Have you used Human Neuropilin-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: