Human CD3 epsilon Antibody
Human CD3 epsilon Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
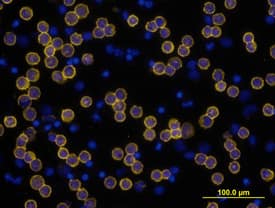 View Larger
View Larger
CD3 epsilon in Human PBMCs. CD3e was detected in immersion fixed human peripheral blood mononuclear cells (PBMCs) using Mouse Anti-Human CD3e Monoclonal Antibody (Catalog # MAB100) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (yellow; Catalog # NL007) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
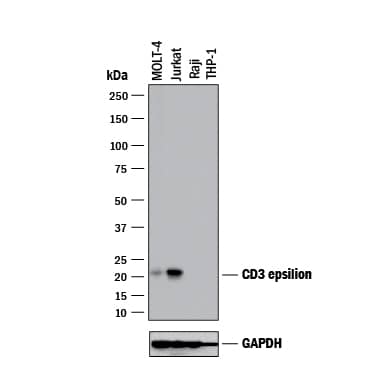 View Larger
View Larger
Detection of Human CD3 epsilon by Western Blot. Western blot shows lysates of MOLT‑4 human acute lymphoblastic leukemia cell line, Jurkat human acute T cell leukemia cell line, Raji human Burkitt's lymphoma cell line (negative control), and THP‑1 human acute monocytic leukemia cell line (negative control). PVDF membrane was probed with 2 µg/mL of Mouse Anti-Human CD3 epsilon Monoclonal Antibody (Catalog # MAB100) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (HAF018). A specific band was detected for CD3 epsilon at approximately 21 kDa (as indicated). GAPDH (MAB5718) is shown as a loading control. This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
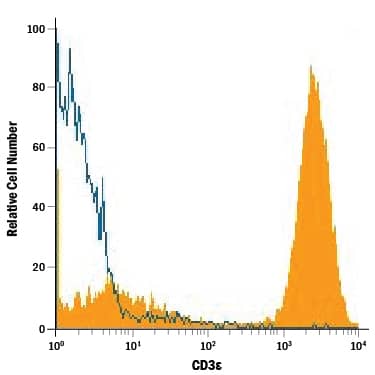 View Larger
View Larger
Detection of CD3 epsilon in Human Lymphocytes by Flow Cytometry. Human peripheral blood lymphocytes were stained with Mouse Anti-Human CD3e Monoclonal Antibody (Catalog # MAB100, filled histogram) or isotype control antibody (Catalog # MAB002, open histogram), followed by Phycoerythrin-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0102B).
 View Larger
View Larger
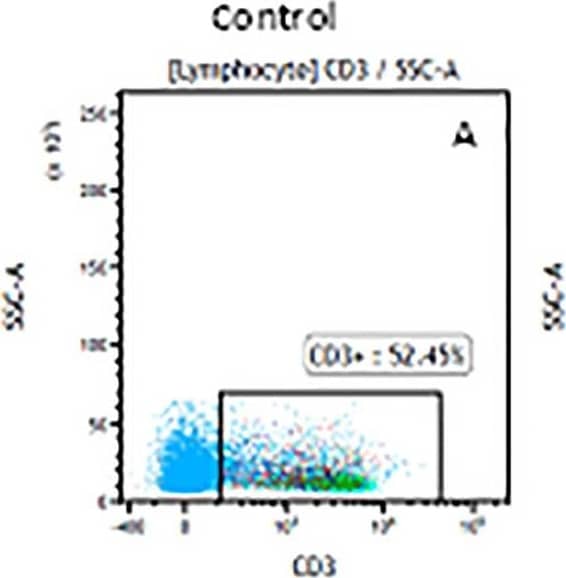
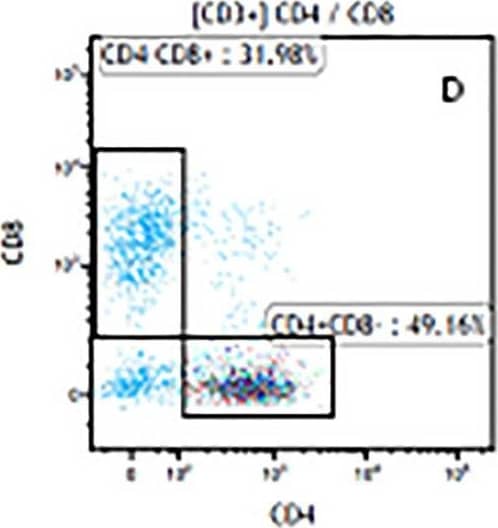
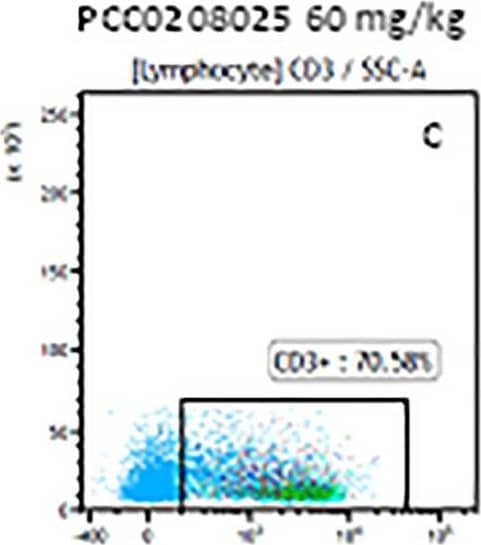
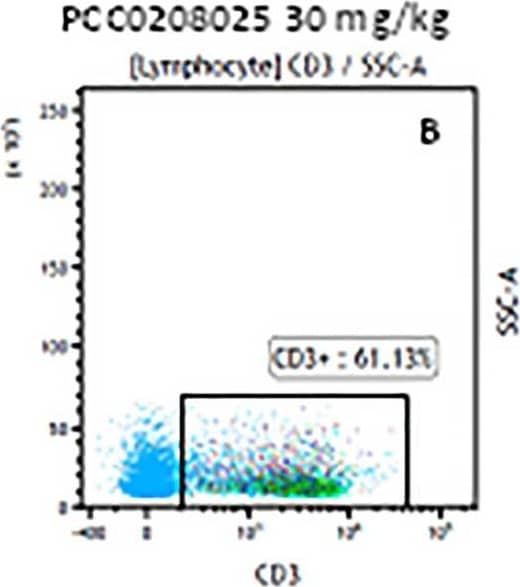
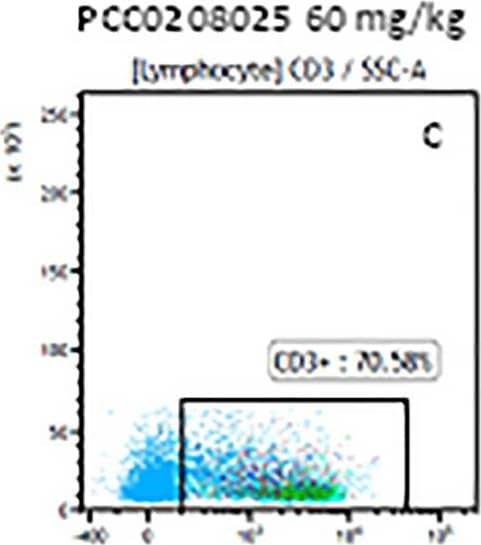
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
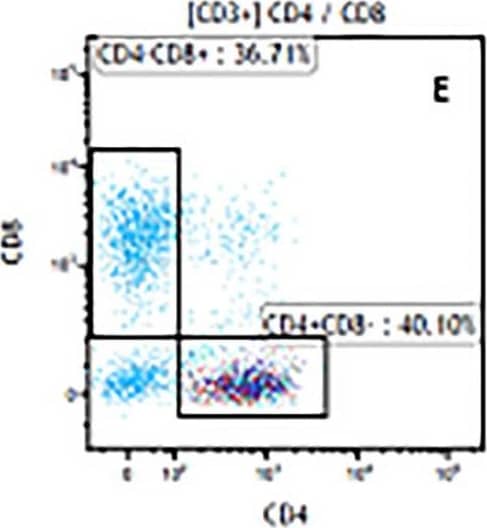 View Larger
View Larger
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD3 epsilon by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
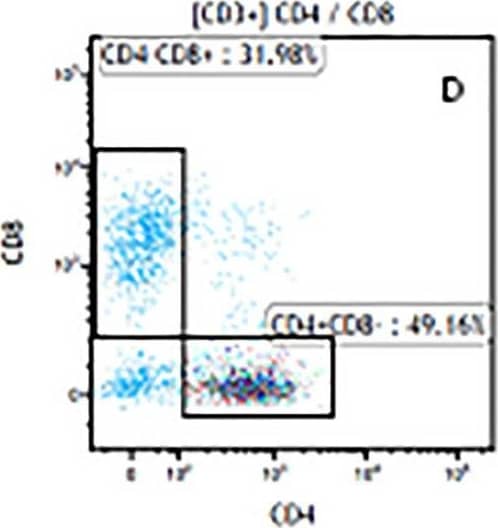
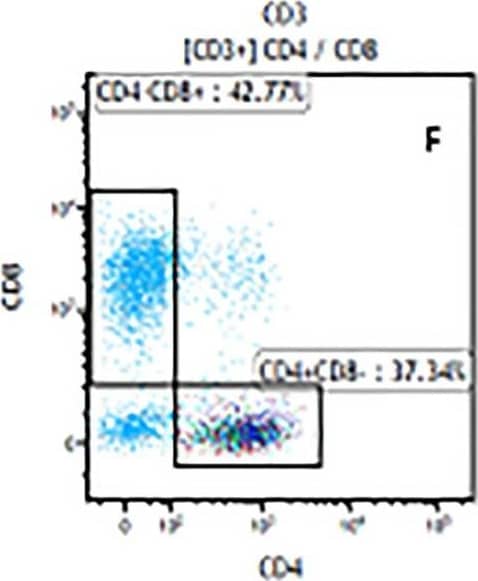
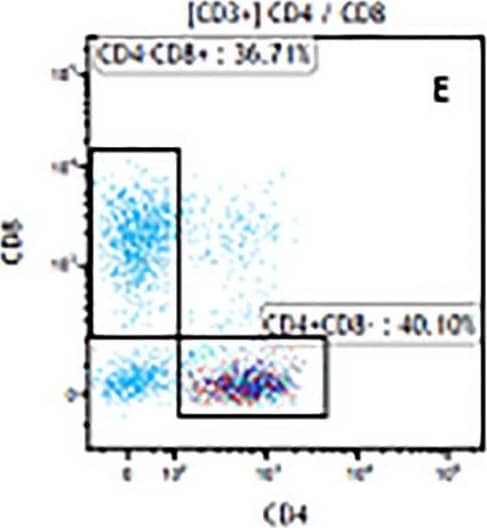
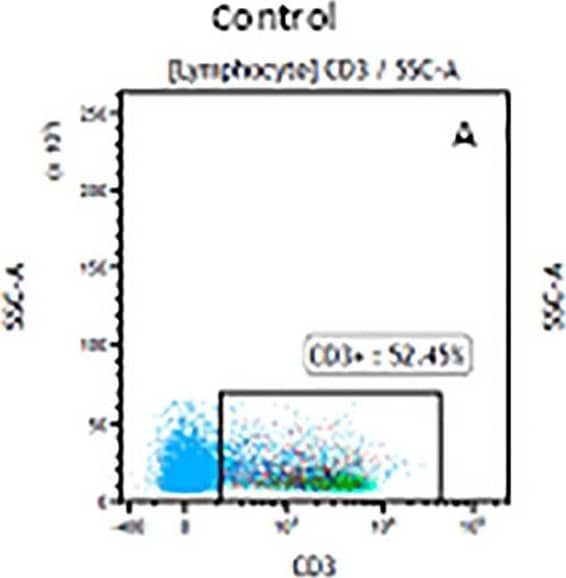
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Human CD3 epsilon Antibody by Flow Cytometry The representative figures for T cell subsets counted by flow cytometry from tumors in B16-F10-bearing mice.The cell counts for CD3+ (A, B and C), CD3+CD4+ (D, E and F), CD3+CD8+ (D, E and F), CD4+CD25+CD127low/- (G, H and I) and CD8+IFN-gamma + (J, K and L) T lymphocytes from mouse tumor were determined by flow cytometry. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/32214351), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD3 epsilon
CD3 epsilon is one of at least four invariant proteins that associate with the variable antigen recognition chains of the T cell receptor and function in signal transduction.
Product Datasheets
Citations for Human CD3 epsilon Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
69
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Comparison study of different indoleamine-2,3 dioxygenase inhibitors from the perspective of pharmacodynamic effects
Authors: Xue Jiang, Xiaopeng Li, Shuang Zheng, Guangying Du, Jinbo Ma, Liming Zhang et al.
Int J Immunopathol Pharmacol
-
Duokines: a novel class of dual-acting co-stimulatory molecules acting in cis or trans
Authors: Sina Fellermeier-Kopf, Friederike Gieseke, Ugur Sahin, Dafne Müller, Klaus Pfizenmaier, Roland E. Kontermann
OncoImmunology
-
Role of natural killer cells in liver transplantation treatment of liver cancer
Authors: Wenbin Ji, Jin Chen, Yuche Mi, Guiliang Wang, Xinjiang Xu, Weizheng Wang
Experimental and Therapeutic Medicine
-
Cytokine-induced killer cell therapy for modulating regulatory T cells in patients with non-small cell lung cancer
Authors: Baodan Yu, Junli Wang, Chen He, Wei Wang, Jianli Tang, Runhui Zheng et al.
Experimental and Therapeutic Medicine
-
Multiple Homozygous Variants in the STING-Encoding TMEM173 Gene in HIV Long-Term Nonprogressors
Authors: SK Nissen, JG Pedersen, M Helleberg, K Kjaer, K Thavachelv, N Obel, M Tolstrup, MR Jakobsen, TH Mogensen
J. Immunol., 2018-04-09;0(0):.
-
Reduced circulating interleukin 35 is associated with enhanced peripheral T cell function in primary biliary cholangitis
Authors: Siqi Liu, Qian Zhang, Mengyao Zhang, Xuejing Zhong, Wudong Wang, Lishuang Wang et al.
Bosnian Journal of Basic Medical Sciences
-
The homodimer interfaces of costimulatory receptors B7 and CD28 control their engagement and pro-inflammatory signaling
Authors: Popugailo, A;Rotfogel, Z;Levy, M;Turgeman, O;Hillman, D;Levy, R;Arad, G;Shpilka, T;Kaempfer, R;
Journal of biomedical science
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference?
Authors: D Peruzzu, ML Dupuis, M Pierdomini, K Fecchi, MC Gagliardi, E Ortona, MT Pagano
International Journal of Molecular Sciences, 2022-08-15;23(16):.
Species: Human
Sample Types: Whole Cells
Applications: Stimulation -
Overexpression of ascitic interleukin-35 induces CD8+ T cell exhaustion in liver cirrhotic patients with spontaneous bacterial peritonitis
Authors: L Yang, S Liu, Q Zhang, S Jia, C Qiu, Z Jin
International immunopharmacology, 2022-03-26;108(0):108729.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Increased IL-6 expression precedes reliable viral detection in the rhesus macaque brain during acute SIV infection
Authors: RM Gopalakris, M Aid, NB Mercado, C Davis, S Malik, E Geiger, V Varner, R Jones, SE Bosinger, C Piedra-Mor, AJ Martinot, DH Barouch, RK Reeves, CS Tan
JCI Insight, 2021-10-22;6(20):.
Species: Primate
Sample Types: Whole Tissue
Applications: IHC -
Glycosaminoglycans bind human IL-27 and regulate its activity
Authors: MC Cavé, S Maillard, K Hildenbran, C Mamelonet, MJ Feige, O Devergne
Eur. J. Immunol., 2020-07-02;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ELISA Development -
Characterization of BAY 1905254, an Immune Checkpoint Inhibitor Targeting the Immunoglobulin-Like Domain Containing Receptor 2 (ILDR2)
Authors: J Huetter, U Gritzan, I Gutcher, WD Doecke, MV Luetke-Eve, S Golfier, HG Roider, AL Frisk, J Hunter, A Pow, A Drake, Z Levine, O Levy, M Azulay, I Barbiro, G Cojocaru, I Vaknin, B Kreft, L Roese
Cancer Immunol Res, 2020-04-20;8(7):895-911.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay, T Cell Activation -
Ex vivo culture of lesional psoriasis skin for pharmacological testing
Authors: ML Tiirikaine, A Woetmann, H Norsgaard, LF Santamaria, P Lovato
J. Dermatol. Sci., 2019-12-27;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: Tissue Culture -
Fisetin, a 3,7,3',4'-Tetrahydroxyflavone Inhibits the PI3K/Akt/mTOR and MAPK Pathways and Ameliorates Psoriasis Pathology in 2D and 3D Organotypic Human Inflammatory Skin Models
Authors: JC Chamcheu, S Esnault, VM Adhami, AL Noll, S Banang-Mbe, T Roy, SS Singh, S Huang, KG Kousoulas, H Mukhtar
Cells, 2019-09-15;8(9):.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Effect of morphine and a low dose of ketamine on the T cells of patients with refractory cancer pain in vitro
Authors: NB Zhou, KG Wang, ZJ Fu
Oncol Lett, 2019-08-16;18(4):4230-4236.
Species: Human
Sample Types: Whole Cells
Applications: Stimulation -
SLAMF6 clustering is required to augment T cell activation
Authors: MA Dragovich, K Adam, M Strazza, AS Tocheva, M Peled, A Mor
PLoS ONE, 2019-06-14;14(6):e0218109.
Species: Human
Sample Types: Whole Cells
Applications: Stimulation -
IL-15-based trifunctional antibody-fusion proteins with costimulatory TNF-superfamily ligands in the single-chain format for cancer immunotherapy
Authors: N Beha, M Harder, S Ring, RE Kontermann, D Müller
Mol. Cancer Ther., 2019-04-30;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer
Authors: F Bensch, EL van der Ve, MN Lub-de Hoo, A Jorritsma-, R Boellaard, IC Kok, SF Oosting, CP Schröder, TJN Hiltermann, AJ van der We, HJM Groen, TC Kwee, SG Elias, JA Gietema, SS Bohorquez, A de Crespig, SP Williams, C Mancao, AH Brouwers, BM Fine, EGE de Vries
Nat. Med., 2018-11-26;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Novel strategies to mimic transmembrane tumor necrosis factor-dependent activation of tumor necrosis factor receptor 2
Authors: R Fischer, J Marsal, C Guttà, SA Eisler, N Peters, JR Bethea, K Pfizenmaie, RE Kontermann
Sci Rep, 2017-07-26;7(1):6607.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue
Authors: C Caër, C Rouault, T Le Roy, C Poitou, J Aron-Wisne, A Torcivia, JC Bichet, K Clément, M Guerre-Mil, S André
Sci Rep, 2017-06-07;7(1):3000.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Interleukin-10-producing LAG3(+) regulatory T cells are associated with disease activity and abatacept treatment in rheumatoid arthritis
Authors: S Nakachi, S Sumitomo, Y Tsuchida, H Tsuchiya, M Kono, R Kato, K Sakurai, N Hanata, Y Nagafuchi, S Tateishi, H Kanda, T Okamura, K Yamamoto, K Fujio
Arthritis Res. Ther., 2017-05-16;19(1):97.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
PLC?1 regulates SDF-1?-induced lymphocyte adhesion and migration to sites of inflammation
Authors: M Strazza, I Azoulay-Al, M Peled, AV Smrcka, EY Skolnik, S Srivastava, A Mor
Proc. Natl. Acad. Sci. U.S.A, 2017-02-17;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
??TCR immunoglobulin constant region domain exchange in human ??TCRs improves TCR pairing without altering TCR gene-modified T cell function
Authors: C Tao, H Shao, W Zhang, H Bo, F Wu, H Shen, S Huang
Mol Med Rep, 2017-02-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts
Authors: Elena Neumann
Matrix Biol., 2016-11-22;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Human trophoblast cells induced MDSCs from peripheral blood CD14(+) myelomonocytic cells via elevated levels of CCL2
Authors: Xun Qu
Cell. Mol. Immunol., 2015-06-01;13(5):615-27.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
T-type calcium channel antagonists, mibefradil and NNC-55-0396 inhibit cell proliferation and induce cell apoptosis in leukemia cell lines.
Authors: Huang W, Lu C, Wu Y, Ouyang S, Chen Y
J Exp Clin Cancer Res, 2015-05-21;34(0):54.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation.
Authors: Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell L, Karoly E, Freeman G, Petkova V, Seth P, Li L, Boussiotis V
Nat Commun, 2015-03-26;6(0):6692.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Direct and indirect effects of immune and central nervous system-resident cells on human oligodendrocyte progenitor cell differentiation.
Authors: Moore C, Cui Q, Warsi N, Durafourt B, Zorko N, Owen D, Antel J, Bar-Or A
J Immunol, 2014-12-10;194(2):761-72.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients.
Authors: Van Belle T, Vanherwegen A, Feyaerts D, De Clercq P, Verstuyf A, Korf H, Gysemans C, Mathieu C
PLoS ONE, 2014-10-03;9(10):e109194.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Optimizations of siRNA design for the activation of gene transcription by targeting the TATA-box motif.
Authors: Fan, Miaomiao, Zhang, Yijun, Huang, Zhuoqion, Liu, Jun, Guo, Xuemin, Zhang, Hui, Luo, Haihua
PLoS ONE, 2014-09-24;9(9):e108253.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Platelets provoke distinct dynamics of immune responses by differentially regulating CD4+ T-cell proliferation.
Authors: Zhu L, Huang Z, Stalesen R, Hansson G, Li N
J Thromb Haemost, 2014-06-27;12(7):1156-65.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients.
Authors: Gao D, Li C, Xie X, Zhao P, Wei X, Sun W, Liu H, Alexandrou A, Jones J, Zhao R, Li J
PLoS ONE, 2014-04-03;9(4):e93886.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The secretion of IL-6 by CpG-ODN-treated cancer cells promotes T-cell immune responses partly through the TLR-9/AP-1 pathway in oral squamous cell carcinoma.
Authors: Ruan M, Thorn K, Liu S, Li S, Yang W, Zhang C, Zhang C
Int J Oncol, 2014-03-21;44(6):2103-10.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis.
Authors: Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G
Cell Stem Cell, 2014-03-06;14(3):342-56.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
IkappaB kinase epsilon targets interferon regulatory factor 1 in activated T lymphocytes.
Authors: Sgarbanti M, Marsili G, Remoli A, Stellacci E, Mai A, Rotili D, Perrotti E, Acchioni C, Orsatti R, Iraci N, Ferrari M, Borsetti A, Hiscott J, Battistini A
Mol Cell Biol, 2014-01-06;34(6):1054-65.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Neuropilin-1 expression characterizes T follicular helper (Tfh) cells activated during B cell differentiation in human secondary lymphoid organs.
Authors: Renand A, Milpied P, Rossignol J, Bruneau J, Lemonnier F, Dussiot M, Coulon S, Hermine O
PLoS ONE, 2013-12-30;8(12):e85589.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1.
Authors: Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, Kukova G, Buhl T, Ikoma A, Buddenkotte J, Soumelis V, Feld M, Alenius H, Dillon S, Carstens E, Homey B, Basbaum A, Steinhoff M
J Allergy Clin Immunol, 2013-12-25;133(2):448-60.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Regulated capture by exosomes of mRNAs for cytoplasmic tRNA synthetases.
Authors: Wang, Feng, Xu, Zhiwen, Zhou, Jie, Lo, Wing-Sze, Lau, Ching-Fu, Nangle, Leslie A, Yang, Xiang-Le, Zhang, Mingjie, Schimmel, Paul
J Biol Chem, 2013-09-03;288(41):29223-8.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
B cells as a therapeutic target for IFN-beta in relapsing-remitting multiple sclerosis.
Authors: Ramgolam VS, Sha Y, Marcus KL, Choudhary N, Troiani L, Chopra M, Markovic-Plese S
J. Immunol., 2011-03-02;186(0):4518.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6.
Authors: Eikawa S, Ohue Y, Kitaoka K
J. Immunol., 2010-11-03;185(11):6734-40.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation.
Authors: Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang EC, Jones SA
FASEB J., 2010-09-08;25(1):409-19.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes.
Authors: Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, Nasorri F, Garcovich S, Traidl-Hoffmann C, Albanesi C, Cavani A
J. Immunol., 2010-03-31;184(9):4880-8.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines.
Authors: Witte K, Gruetz G, Volk HD, Looman AC, Asadullah K, Sterry W, Sabat R, Wolk K
Genes Immun., 2009-10-01;10(8):702-14.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Cutaneous lymphocyte-associated antigen (CLA) T cells up-regulate P-selectin ligand expression upon their activation.
Authors: Ni Z, Walcheck B
Clin. Immunol., 2009-08-08;133(2):257-64.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Phase I therapeutic trial of the HIV-1 Tat protein and long term follow-up.
Authors: Longo O, Tripiciano A, Fiorelli V, Bellino S, Scoglio A, Collacchi B, Alvarez MJ, Francavilla V, Arancio A, Paniccia G, Lazzarin A, Tambussi G, Din CT, Visintini R, Narciso P, Antinori A, D'Offizi G, Giulianelli M, Carta M, Di Carlo A, Palamara G, Giuliani M, Laguardia ME, Monini P, Magnani M, Ensoli F, Ensoli B
Vaccine, 2009-02-07;27(25):3306-12.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells.
Authors: Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R
Blood, 2009-02-06;113(24):6120-7.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Human bronchial intraepithelial T cells produce interferon-gamma and stimulate epithelial cells.
Authors: Hirosako S, Goto E, Fujii K, Tsumori K, Hirata N, Tsumura S, Kamohara H, Kohrogi H
Clin. Exp. Immunol., 2008-11-26;155(2):266-74.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Interactions of T cells with fibroblast-like synoviocytes: role of the B7 family costimulatory ligand B7-H3.
Authors: Tran CN, Thacker SG, Louie DM, Oliver J, White PT, Endres JL, Urquhart AG, Chung KC, Fox DA
J. Immunol., 2008-03-01;180(5):2989-98.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-Fr, IHC-P -
Preferential migration of T regulatory cells induced by IL-16.
Authors: McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, Cruikshank W
J. Immunol., 2007-11-15;179(10):6439-45.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Interaction between transmembrane TNF and TNFR1/2 mediates the activation of monocytes by contact with T cells.
Authors: Rossol M, Meusch U, Pierer M, Kaltenhauser S, Hantzschel H, Hauschildt S, Wagner U
J. Immunol., 2007-09-15;179(6):4239-48.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells.
Authors: Bayry J, Triebel F, Kaveri SV, Tough DF
J. Immunol., 2007-04-01;178(7):4184-93.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Flow-cytometric analysis of cytokine production in human paracoccidioidomycosis.
Authors: Mamoni RL, Blotta MH
Cytokine, 2006-10-11;35(3):207-16.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
A reproducible method for the enumeration of functional (cytokine producing) versus non-functional peptide-specific cytotoxic T lymphocytes in human peripheral blood.
Authors: Markovic SN, Nevala WK, Uhl CB, Celis E, McKean DJ
Clin. Exp. Immunol., 2006-09-01;145(3):438-47.
Species: Human
Sample Types:
Applications: Binding Assay -
B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease.
Authors: Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA
Am. J. Pathol., 2006-09-01;169(3):987-98.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: IHC-Fr, Immunoprecipitation -
Up-regulation of macrophage migration inhibitory factor in infants with acute neonatal necrotizing enterocolitis.
Authors: Ren Y, Lin CL, Li Z, Chen XY, Huang X, Lui V, Nicholls J, Lan HY, Tam PK
Histopathology, 2005-06-01;46(6):659-67.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay.
Authors: Ogilvie RL, Abelson M, Hau HH, Vlasova I, Blackshear PJ, Bohjanen PR
J. Immunol., 2005-01-15;174(2):953-61.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone.
Authors: Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ, Lee FY
J. Orthop. Res., 2005-01-01;23(1):203-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
IL-22 increases the innate immunity of tissues.
Authors: Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R
Immunity, 2004-08-01;21(2):241-54.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Induction of lysosomal and plasma membrane-bound sialidases in human T-cells via T-cell receptor.
Authors: Wang P, Zhang J, Bian H, Wu P, Kuvelkar R, Kung TT, Crawley Y, Egan RW, Billah MM
Biochem. J., 2004-06-01;380(0):425-33.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis.
Authors: Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J
PLoS Biol., 2004-01-20;2(1):E6.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
In vitro differentiation from naive to mature E-selectin binding CD4 T cells: acquisition of skin-homing properties occurs independently of cutaneous lymphocyte antigen expression.
Authors: Takahashi R, Mizukawa Y, Yamazaki Y, Hayakawa K, Hayakawa J, Kudo A, Shiohara T
J. Immunol., 2003-12-01;171(11):5769-77.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial fibroblasts by hypoxia and reoxygenation: potential role in rheumatoid arthritis.
Authors: Han MK, Kim JS, Park BH, Kim JR, Hwang BY, Lee HY, Song EK, Yoo WH
J. Leukoc. Biol., 2003-04-01;73(4):525-9.
Species: Feline
Sample Types: Whole Cells
Applications: Flow Cytometry -
TOM1L is involved in a novel signaling pathway important for the IL-2 production in Jurkat T cells stimulated by CD3/CD28 co-ligation
Authors: Ahmed Elmarghani, Hanan Abuabaid, Peter Kjellen
Mediators of Inflammation
-
Fc-based Duokines: dual-acting costimulatory molecules comprising TNFSF ligands in the single-chain format fused to a heterodimerizing Fc (scDk-Fc)
Authors: Nadine Aschmoneit, Katharina Kocher, Martin Siegemund, Martina S. Lutz, Lennart Kühl, Oliver Seifert et al.
OncoImmunology
-
Interleukin-25 initiates Th2 differentiation of human CD4(+) T cells and influences expression of its own receptor
Authors: Graeme Bredo, Jessica Storie, Nami Shrestha Palikhe, Courtney Davidson, Alexis Adams, Harissios Vliagoftis et al.
Immunity, Inflammation and Disease
-
RAB11FIP5-Deficient Mice Exhibit Cytokine-Related Transcriptomic Signatures
Authors: Dapeng Li, Todd Bradley, Derek W. Cain, Isabela Pedroza-Pacheco, Maria Aggelakopoulou, Robert Parks et al.
ImmunoHorizons
-
Influence of antigen density and immunosuppressive factors on tumor-targeted costimulation with antibody-fusion proteins and bispecific antibody-mediated T cell response
Authors: Sabrina Sapski, Nadine Beha, Roland E. Kontermann, Dafne Müller
Cancer Immunology, Immunotherapy
-
NaHCO3 enhances the antitumor activities of cytokine-induced killer cells against hepatocellular carcinoma HepG2 cells
Authors: Ya Hong Yuan, Chun Fang Zhou, Jiang Yuan, Li Liu, Xing Rong Guo, Xiao Li Wang et al.
Oncology Letters
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CD3 epsilon Antibody
Average Rating: 5 (Based on 4 Reviews)
Have you used Human CD3 epsilon Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:






