Mouse LYVE-1 Antibody Summary
Ala24-Thr234
Accession # Q8BHC0
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse LYVE‑1 by Western Blot. Western blot shows lysate of bEnd.3 mouse endothelioma cell line. PVDF membrane was probed with 2 µg/mL of Rat Anti-Mouse LYVE-1 Monoclonal Antibody (Catalog # MAB2125) followed by HRP-conjugated Anti-Rat IgG Secondary Antibody (Catalog # HAF005). A specific band was detected for LYVE-1 at approximately 65 kDa (as indicated). This experiment was conducted under non-reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of LYVE‑1 in bEnd.3 Mouse Cell Line by Flow Cytometry. bEnd.3 mouse endothelioma cell line was stained with Rat Anti-Mouse LYVE-1 Monoclonal Antibody (Catalog # MAB2125, filled histogram) or isotype control antibody (Catalog # MAB006, open histogram), followed by Phycoerythrin-conjugated Anti-Rat IgG Secondary Antibody (Catalog # F0105B).
 View Larger
View Larger
LYVE-1 in NS0 mouse myeloma cell line transfected with mouse LYVE-1. LYVE-1 was detected in immersion fixed NS0 mouse myeloma cell line transfected with mouse LYVE-1 (positive control) and wild type NS0 mouse myeloma cell line (negative control) using Rat Anti-Mouse LYVE-1 Monoclonal Antibody (Catalog # MAB2125) at 8 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (red; NL013) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
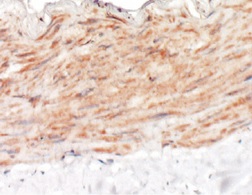
LYVE-1 in Mouse Intestine. LYVE-1 was detected in immersion fixed frozen sections of mouse intestine tissue using Rat Anti-Mouse LYVE-1 Monoclonal Antibody (Catalog # MAB2125) at 15 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC005). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to endothelial cells in lymphatic vessels. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: LYVE-1
Lymphatic vessel endothelial hyaluronan (HA) receptor-1 (LYVE-1) is a recently identified receptor of HA, a linear high molecular weight polymer composed of alternating units of D-glucuronic acid and N-acetyl-D-glucosamine. HA is found in the extracellular matrix of most animal tissues and in body fluids. It modulates cell behavior and functions during tissue remodeling, development, homeostasis, and disease (1). The turnover of HA (several grams/day in humans) occurs primarily in the lymphatics and liver, the two major clearance systems that catabolize approximately 85% and 15% of HA, respectively (1-3). LYVE-1 shares 41% homology with the other known HA receptor, CD44 (4). The homology between the two proteins increases to 61% within the HA binding domain. The HA binding domain, known as the link module, is a common structural motif found in other HA binding proteins such as link protein, aggrecan and versican (1, 5). Human and mouse LYVE-1 share 69% amino acid sequence identity.
LYVE-1 is primarily expressed on both the luminal and abluminal surfaces of lymphatic vessels (4, 5). In addition, LYVE-1 is also present in normal hepatic blood sinusoidal endothelial cells (6). LYVE-1 mediates the endocytosis of HA and may transport HA from tissue to lymph by transcytosis, delivering HA to lymphatic capillaries for removal and degradation in the regional lymph nodes (5, 7, 8). Because of its restricted expression patterns, LYVE-1, along with other lymphatic proteins such as VEGF R3, podoplanin and the homeobox protein propero-related (Prox-1), constitute a set of markers useful for distinguishing between lymphatic and blood microvasculature (4, 5, 9-11).
- Knudson, C.B. and W. Knudson (1993) FASEB J. 7:1233.
- Evered, D. and J. Whelan (1989) Ciba Found. Symp. 143:1.
- Laurent, T.C. and J.R.F. Fraser (1992) FASEB J. 6:2397.
- Banerji, S. et al. (1999) J. Cell Biol. 144:789.
- Prevo, R. et al. (2001) J. Biol. Chem. 276:19420.
- Carreira, C.M. et al. (2001) Cancer Research 61:8079.
- Jackson, D.J. et al. (2001) Trends Immunol. 22:317.
- Zhou, B. et al. (2000) J. Biol. Chem. 275:37733.
- Achen, M. et al. (1998) Proc. Natl. Acad. Sci. USA 95:548.
- Breiteneder-Gellef, S. et al. (1999) Am. J. Pathol. 154:385.
- Wiggle, J.T. and G. Oliver (1999) Cell 98:769.
Product Datasheets
Citations for Mouse LYVE-1 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
40
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Heterogeneity in VEGFR3 levels drives lymphatic vessel hyperplasia through cell-autonomous and non-cell-autonomous mechanisms
Authors: Y Zhang, MH Ulvmar, L Stanczuk, I Martinez-C, M Frye, K Alitalo, T Mäkinen
Nat Commun, 2018-04-03;9(1):1296.
-
Hormone Therapy: A Potential Risk Factor Affecting Survival and Functional Restoration of Transplanted Lymph Nodes
Authors: Dong Dong, Heng Wang, Liang Chen, Wei Wang, Tianyi Liu
Frontiers in Pharmacology
-
Self-organized and directed branching results in optimal coverage in developing dermal lymphatic networks
Authors: Uçar, MC;Hannezo, E;Tiilikainen, E;Liaqat, I;Jakobsson, E;Nurmi, H;Vaahtomeri, K;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Imaging Blood Vessels and Lymphatics in Mouse Trachea Wholemounts
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Ileitis-associated tertiary lymphoid organs originate at lymphatic valves and obstruct mesenteric lymph flow in response to tumor necrosis factor
Authors: Rafael S. Czepielewski, Emma C. Erlich, Emily J. Onufer, Shannon Young, Brian T. Saunders, Yong-Hyun Han et al.
Immunity
-
Vegfr3-tdTomato, a reporter mouse for microscopic visualization of lymphatic vessel by multiple modalities
Authors: E Redder, N Kirschnick, S Bobe, R Hägerling, NR Hansmeier, F Kiefer
PLoS ONE, 2021-09-20;16(9):e0249256.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Potential functions of embryonic cardiac macrophages in angiogenesis, lymphangiogenesis and extracellular matrix remodeling
Authors: Grzegorz Gula, Sławomir Rumiński, Justyna Niderla-Bielińska, Agnieszka Jasińska, Ewelina Kiernozek, Ewa Jankowska-Steifer et al.
Histochemistry and Cell Biology
-
EphrinB2-EphB4 signalling provides Rho-mediated homeostatic control of lymphatic endothelial cell junction integrity
Authors: Maike Frye, Simon Stritt, Henrik Ortsäter, Magda Hernandez Vasquez, Mika Kaakinen, Andres Vicente et al.
eLife
-
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: In Vivo
Applications: ELISA Detection, Labeling -
The lymph node stromal laminin alpha 5 shapes alloimmunity
Authors: L Li, MW Shirkey, T Zhang, Y Xiong, W Piao, V Saxena, C Paluskievi, YS Lee, N Toney, BM Cerel, Q Li, T Simon, KD Smith, KL Hippen, BR Blazar, R Abdi, JS Bromberg
J. Clin. Invest., 2020-05-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Foliate Lymphoid Aggregates as Novel Forms of Serous Lymphocyte Entry Sites of Peritoneal B Cells and High-Grade B Cell Lymphomas
Authors: X Jia, F Gábris, Ó Jacobsen, G Bedics, B Botz, Z Helyes, Z Kellermaye, D Vojkovics, G Berta, N Nagy, Z Jakus, P Balogh
J. Immunol., 2019-11-25;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Imaging Lymphatics in Mouse Lungs
Authors: Peter Baluk, Donald M. McDonald
Methods in Molecular Biology
-
Comparative Transcriptomic Analysis Identifies a Range of Immunologically Related Functional Elaborations of Lymph Node Associated Lymphatic and Blood Endothelial Cells
Authors: Stella J. Berendam, Alexander F. Koeppel, Nicole R. Godfrey, Sherin J. Rouhani, Amber N. Woods, Anthony B. Rodriguez et al.
Frontiers in Immunology
-
Single-Cell Analysis Reveals Heterogeneity of High Endothelial Venules and Different Regulation of Genes Controlling Lymphocyte Entry to Lymph Nodes
Authors: K Veerman, C Tardiveau, F Martins, J Coudert, JP Girard
Cell Rep, 2019-03-12;26(11):3116-3131.e5.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Cyp1b1 expression impacts the angiogenic and inflammatory properties of liver sinusoidal endothelial cells
Authors: J Falero-Per, YS Song, Y Zhao, L Teixeira, CM Sorenson, N Sheibani
PLoS ONE, 2018-10-29;13(10):e0206756.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
BMP9, but not BMP10, acts as a quiescence factor on tumor growth, vessel normalization and metastasis in a mouse model of breast cancer
Authors: M Ouarné, C Bouvard, G Boneva, C Mallet, J Ribeiro, A Desroches-, E Soleilhac, E Tillet, O Peyruchaud, S Bailly
J. Exp. Clin. Cancer Res., 2018-08-30;37(1):209.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program
Authors: M Frye, A Taddei, C Dierkes, I Martinez-C, M Fielden, H Ortsäter, J Kazenwadel, DP Calado, P Ostergaard, M Salminen, L He, NL Harvey, F Kiefer, T Mäkinen
Nat Commun, 2018-04-17;9(1):1511.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
T Cells in Nonlymphoid Tissues Give Rise to Lymph-Node-Resident Memory T Cells
Authors: LK Beura, S Wijeyesing, EA Thompson, MG Macchietto, PC Rosato, MJ Pierson, JM Schenkel, JS Mitchell, V Vezys, BT Fife, S Shen, D Masopust
Immunity, 2018-02-20;48(2):327-338.e5.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Development and plasticity of meningeal lymphatic vessels
Authors: Salli Antila, Sinem Karaman, Harri Nurmi, Mikko Airavaara, Merja H. Voutilainen, Thomas Mathivet et al.
Journal of Experimental Medicine
-
In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors
Authors: T Torcellan, HR Hampton, J Bailey, M Tomura, R Brink, T Chtanova
Proc. Natl. Acad. Sci. U.S.A., 2017-05-15;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1
Authors: LA Johnson, S Banerji, W Lawrance, U Gileadi, G Prota, KA Holder, YM Roshorm, T Hanke, V Cerundolo, NW Gale, DG Jackson
Nat. Immunol., 2017-05-15;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A reverse signaling pathway downstream of Sema4A controls cell migration via Scrib
Authors: Tianliang Sun
J. Cell Biol, 2016-12-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Characterization of the Expression and Function of the C-Type Lectin Receptor CD302 in Mice and Humans Reveals a Role in Dendritic Cell Migration
Authors: Tsun-Ho Lo
J Immunol, 2016-06-17;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
A micro-sterile inflammation array as an adjuvant for influenza vaccines.
Authors: Wang J, Shah D, Chen X, Anderson R, Wu M
Nat Commun, 2014-07-18;5(0):4447.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways.
Authors: Wu M, Du Y, Liu Y, He Y, Yang C, Wang W, Gao F
PLoS ONE, 2014-03-25;9(3):e92857.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Fusing VE-cadherin to alpha-catenin impairs fetal liver hematopoiesis and lymph but not blood vessel formation.
Authors: Dartsch N, Schulte D, Hagerling R, Kiefer F, Vestweber D
Mol Cell Biol, 2014-02-24;34(9):1634-48.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle.
Authors: Thorek D, Ulmert D, Diop N, Lupu M, Doran M, Huang R, Abou D, Larson S, Grimm J
Nat Commun, 2014-01-01;5(0):3097.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation
Authors: Sandrine Levet, Delphine Ciais, Galina Merdzhanova, Christine Mallet, Teresa A. Zimmers, Se-Jin Lee et al.
Blood
-
Mouse lymphatic endothelial cell targeted probes: anti-LYVE-1 antibody-based magnetic nanoparticles
Authors: Qiu Guo, Yi Ke Xu, Ke Ke Ren, Ke WenGe Sun, WenGe Yi Liu
International Journal of Nanomedicine
-
A role for LFA-1 in delaying T-lymphocyte egress from lymph nodes.
Authors: Reichardt, Peter, Patzak, Irene, Jones, Kristian, Etemire, Eloho, Gunzer, Matthias, Hogg, Nancy
EMBO J, 2013-02-26;32(6):829-43.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
FTY720 blocks egress of T cells in part by abrogation of their adhesion on the lymph node sinus.
Authors: Zhi L, Kim P, Thompson BD, Pitsillides C, Bankovich AJ, Yun SH, Lin CP, Cyster JG, Wu MX
J. Immunol., 2011-07-25;187(5):2244-51.
Species: Mouse
Sample Types: In Vivo
Applications: Intravital Microscopy, Intravital Microscopy -
TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice.
Authors: Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM
J. Clin. Invest., 2009-09-14;119(10):2954-64.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth.
Authors: Cho WG, Albuquerque RJ, Kleinman ME, Tarallo V, Greco A, Nozaki M, Green MG, Baffi JZ, Ambati BK, De Falco M, Alexander JS, Brunetti A, De Falco S, Ambati J
Proc. Natl. Acad. Sci. U.S.A., 2009-04-09;106(17):7137-42.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis.
Authors: Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH
Immunity, 2009-02-19;30(3):434-46.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
CXCR4-gp120-IIIB interactions induce caspase-mediated apoptosis of prostate cancer cells and inhibit tumor growth.
Authors: Singh S, Bond VC, Powell M, Singh UP, Bumpers HL, Grizzle WE, Lillard JW
Mol. Cancer Ther., 2009-01-01;8(1):178-84.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells.
Authors: Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG
Nat. Immunol., 2008-12-07;10(1):58-65.
Species: Mouse
Sample Types: In Vivo, Whole Tissue
Applications: IHC-Fr, Neutralization -
S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress.
Authors: Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG
Immunity, 2007-12-27;28(1):122-33.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: Flow Cytometry, IHC-Fr -
CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells.
Authors: Kabashima K, Shiraishi N, Sugita K, Mori T, Onoue A, Kobayashi M, Sakabe J, Yoshiki R, Tamamura H, Fujii N, Inaba K, Tokura Y
Am. J. Pathol., 2007-09-06;171(4):1249-57.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells.
Authors: Phan TG, Grigorova I, Okada T, Cyster JG
Nat. Immunol., 2007-07-29;8(9):992-1000.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor.
Authors: Backhed F, Crawford PA, O'Donnell D, Gordon JI
Proc. Natl. Acad. Sci. U.S.A., 2007-01-03;104(2):606-11.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse LYVE-1 Antibody
Average Rating: 5 (Based on 3 Reviews)
Have you used Mouse LYVE-1 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
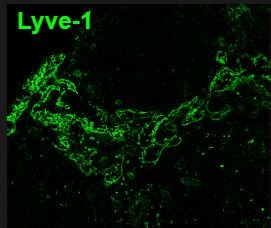
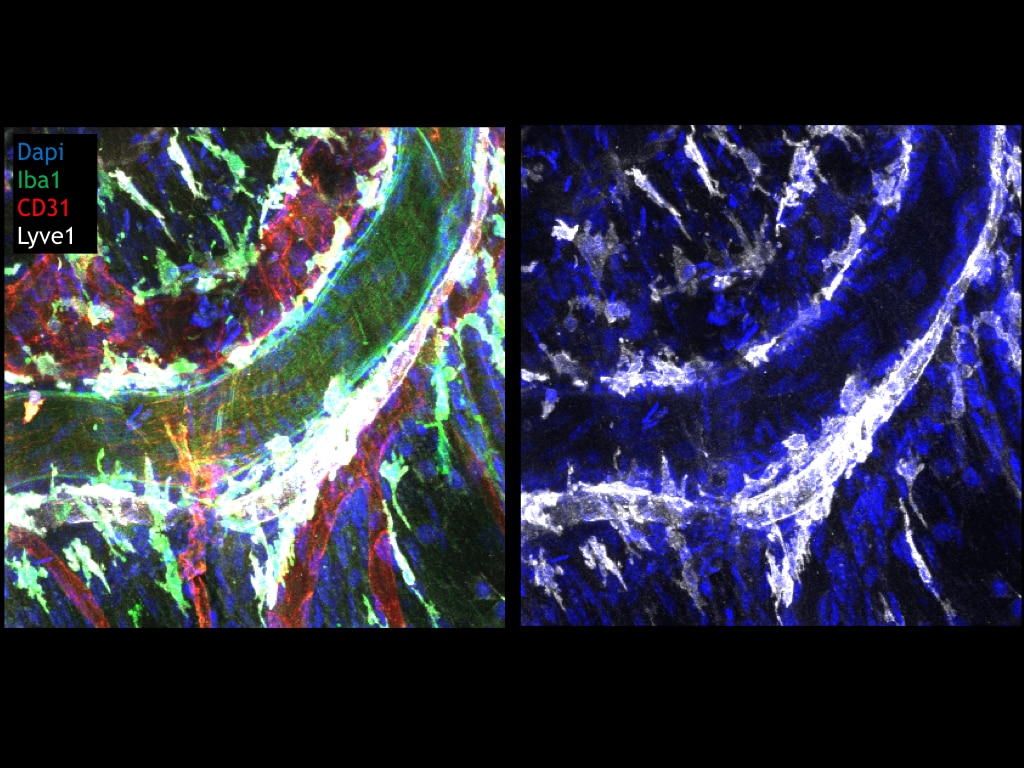
LN were fixed in 4% PFA, sectioned and blocked/permeabilized with 0.1% Tx-100, 0.01% NaN3, 1% FBS. Sections were stained overnight (MAB2125) at a dilution of 1:200. Antibody was detected using a secondary FITC-anti rat (1:500).