Human VE-Cadherin Antibody Summary
no cross‑reactivity with recombinant human (rh) Cadherin-17 or rhP-Cadherin is observed.
Asp48-Gln593
Accession # P33151
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human VE‑Cadherin by Western Blot. Western blot shows lysate of HUVEC human umbilical vein endothelial cells. PVDF membrane was probed with 1 µg/mL of Mouse Anti-Human VE-Cadherin Monoclonal Antibody (Catalog # MAB9381) followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # HAF018). A specific band was detected for VE-Cadherin at approximately 125 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
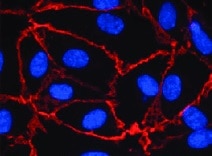
VE‑Cadherin in HUVEC Cells. VE-Cadherin was detected in immersion fixed HUVEC cells using Mouse Anti-Human VE-Cadherin Monoclonal Antibody (Catalog # MAB9381) at 0.5 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (red; Catalog # NL007) and counterstained with DAPI (blue). Specific staining was localized to plasma membrane. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Detection of Human VE-Cadherin by Flow Cytometry Immortalized HAECs retain phenotypic and functional characteristics of primary cells. (A) (Top) Immortalized HAEC confluent monolayers stained with CD31-AF488 antibody (green) and DAPI (blue). (Bottom) Flow cytometry histogram plots of primary or immortalized HAECs labeled with CD31-AF488 antibody. Secondary antibody only (dashed line) and nonstained cells (dotted line) were used as negative controls. (B) (Top) Immortalized HAEC confluent monolayers stained with VE-cadherin-AF488 (green) antibody, Acti-Stain 555 phalloidin (red), and DAPI (blue). (Bottom) Flow cytometry histogram plots of immortalized HAECs labeled with VE-cadherin-AF488 and ZO-1-AF647 antibodies. Secondary antibody only (dashed line) was used as a negative control. (C) Tube forming assay of primary (top) and immortalized (bottom) HAECs at 0 h and 8 h after seeding in Matrigel-coated wells. (D) Acetylated low-density lipoprotein (Ac-LDL) uptake assay of primary HAECs (top), immortalized HAECs (middle), and fibroblasts (negative control; bottom) treated with fluorescently labeled Ac-LDL (red) for 2.5 h and stained with DAPI (blue). (E) IL-8 detection in culture supernatants 24 h after treatment of primary or immortalized HAECs with increasing concentrations of TNF-alpha, IFN-gamma, or IL-6. (F) IL-8 detection in culture supernatants 24 h after treatment of immortalized HAECs with increasing concentrations of IL-2 or IL-1. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29229737), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VE-Cadherin
Vascular endothelial (VE)-cadherin (VE-CAD), also called 7B4 and cadherin‑5 (CDH5), is a member of the cadherin family of cell adhesion molecules. Cadherins are calcium‑dependent transmembrane proteins which bind to one another in a homophilic manner. On their cytoplasmic side, they associate with the three catenins, alpha, beta, and gamma (plakoglobin). This association links the cadherin protein to the cytoskeleton. Without association with the catenins, the cadherins are non-adhesive. Cadherins play a role in development, specifically in tissue formation. They may also help to maintain tissue architecture in the adult. VE-cadherin has been shown to play important roles in vasculogenesis and angiogenesis. VE-cadherin is a classical cadherin molecule. Classical cadherins consist of a large extracellular domain which contains DXD and DXNDN repeats responsible for mediating calcium-dependent adhesion, a single-pass transmembrane domain, and a short carboxy-terminal cytoplasmic domain responsible for interacting with the catenins. Human VE-cadherin is a 784 amino acid (aa) residue protein with a 25 aa signal sequence and a 759 aa propeptide. The mature protein begins at amino acid 48 and has a 546 aa extracellular domain, a 27 aa transmembrane domain, and a 164 aa cytoplasmic domain. The human and mouse mature VE-cadherin proteins share approximately 74% homology.
- Shimoyama, Y. et al. (1989) J. Cell Biol. 109:1787.
- Bussemakers, M.J.G. et al. (1993) Mol. Biol. Reports 17:123.
- Overduin, M. et al. (1995) Science 267:386.
- Takeichi, M. (1991) Science 251:1451.
- Nose, A. et al. (1987) EMBO J. 6:3655.
- Carmeliet, P. et al. (1999) Cell 98:147.
- Gory-Faure, S. et al. (1999) Development 126:2093.
Product Datasheets
Citations for Human VE-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
28
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The Multi-Kinase Inhibitor RepSox Enforces Barrier Function in the Face of Both VEGF and Cytokines
Authors: Lietuvninkas, L;Baccouche, B;Kazlauskas, A;
Biomedicines
Species: Human
Sample Types: Whole Cells
Applications: ICC -
PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing
Authors: Eulashini Chuntharpursat-Bon, Oleksandr V. Povstyan, Melanie J. Ludlow, David J. Carrier, Marjolaine Debant, Jian Shi et al.
Communications Biology
-
Mesenchymal stem cells support human vascular endothelial cells to form vascular sprouts in human platelet lysate-based matrices
Authors: S Summer, E Rossmanith, M Pasztorek, C Fiedler, M Gröger, S Rauscher, V Weber, MB Fischer
PLoS ONE, 2022-12-15;17(12):e0278895.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Hypoxia induces purinergic receptor signaling to disrupt endothelial barrier function
Authors: Somasundaram Raghavan, Masuma Akter Brishti, Daniel Mohr Collier, M. Dennis Leo
Frontiers in Physiology
-
FSAP Protects against Histone-Mediated Increase in Endothelial Permeability In Vitro
Authors: XY Cui, B Stavik, B Thiede, PM Sandset, SM Kanse
International Journal of Molecular Sciences, 2022-11-08;23(22):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Endothelial deletion of TBK1 contributes to BRB dysfunction via CXCR4 phosphorylation suppression
Authors: Bowen Zhao, Yueqi Ni, Hong Zhang, Yin Zhao, Lu Li
Cell Death Discovery
-
A combined human gastruloid model of cardiogenesis and neurogenesis
Authors: Olmsted ZT, Paluh JL.
iScience
-
Spatiotemporally controlled, aptamers-mediated growth factor release locally manipulates microvasculature formation within engineered tissues
Authors: Deepti Rana, Ajoy Kandar, Nasim Salehi-Nik, Ilyas Inci, Bart Koopman, Jeroen Rouwkema
Bioactive Materials
-
The LINC complex is required for endothelial cell adhesion and adaptation to shear stress and cyclic stretch
Authors: Kevin B. Denis, Jolene I. Cabe, Brooke E. Danielsson, Katie V. Tieu, Carl R. Mayer, Daniel E. Conway
Molecular Biology of the Cell
-
Differential Responses of Transplanted Stem Cells to Diseased Environment Unveiled by a Molecular NIR-II Cell Tracker
Authors: Hao Chen, Huaxiao Yang, Chen Zhang, Si Chen, Xin Zhao, Mark Zhu et al.
Research (Wash D C)
-
Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging
Authors: Nicholas E. Propson, Ethan R. Roy, Alexandra Litvinchuk, Jörg Köhl, Hui Zheng
Journal of Clinical Investigation
-
Lowering the increased intracellular pH of human-induced pluripotent stem cell-derived endothelial cells induces formation of mature Weibel-Palade bodies
Authors: Gesa L. Tiemeier, Rozemarijn de Koning, Gangqi Wang, Sarantos Kostidis, Rosalie G. J. Rietjens, Wendy M. P. J. Sol et al.
Stem Cells Translational Medicine
-
Fibronectin Adsorption on Electrospun Synthetic Vascular Grafts Attracts Endothelial Progenitor Cells and Promotes Endothelialization in Dynamic In Vitro Culture
Authors: R Daum, D Visser, C Wild, L Kutuzova, M Schneider, G Lorenz, M Weiss, S Hinderer, UA Stock, M Seifert, K Schenke-La
Cells, 2020-03-23;9(3):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN
Authors: Marilina Antonelou, Erik Michaëlsson, Rhys D.R. Evans, Chun Jing Wang, Scott R. Henderson, Lucy S.K. Walker et al.
Journal of the American Society of Nephrology
-
Adipose tissue–derived stromal cells’ conditioned medium modulates endothelial‐mesenchymal transition induced by IL‐1 beta /TGF‐ beta 2 but does not restore endothelial function
Authors: Tácia Tavares Aquinas Liguori, Gabriel Romero Liguori, Luiz Felipe Pinho Moreira, Martin Conrad Harmsen
Cell Proliferation
-
Complement activation on neutrophils initiates endothelial adhesion and extravasation
Authors: Antonina Akk, Luke E. Springer, Lihua Yang, Samantha Hamilton-Burdess, John D. Lambris, Huimin Yan et al.
Molecular Immunology
-
Heparanase-2 protects from LPS-mediated endothelial injury by inhibiting TLR4 signalling
Authors: Y Kiyan, S Tkachuk, K Kurselis, N Shushakova, K Stahl, D Dawodu, R Kiyan, B Chichkov, H Haller
Sci Rep, 2019-09-19;9(1):13591.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Human Fibrinogen for Maintenance and Differentiation of Induced Pluripotent Stem Cells in Two Dimensions and Three Dimensions
Authors: Jarel K. Gandhi, Travis Knudsen, Matthew Hill, Bhaskar Roy, Lori Bachman, Cynthia Pfannkoch-Andrews et al.
Stem Cells Translational Medicine
-
Human pre-valvular endocardial cells derived from pluripotent stem cells recapitulate cardiac pathophysiological valvulogenesis
Authors: T Neri, E Hiriart, PP van Vliet, E Faure, RA Norris, B Farhat, B Jagla, J Lefrancois, Y Sugi, T Moore-Morr, S Zaffran, RS Faustino, AC Zambon, JP Desvignes, D Salgado, RA Levine, JL de la Pomp, A Terzic, SM Evans, R Markwald, M Pucéat
Nat Commun, 2019-04-26;10(1):1929.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis
Authors: BL Ecker, A Kaur, SM Douglass, MR Webster, FV Almeida, GE Marino, AJ Sinnamon, MG Neuwirth, GM Alicea, A Ndoye, M Fane, X Xu, MS Sim, GB Deutsch, MB Faries, GC Karakousis, AT Weeraratna
Cancer Discov, 2018-10-02;9(1):82-95.
Species: Human
Sample Types: Whole Cells, Whole Tissue
Applications: ICC -
Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages
Authors: J Li, F Xing, F Chen, L He, KF So, Y Liu, J Xiao
Cell Transplant, 2018-06-13;0(0):9636897187803.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
The Superantigen Toxic Shock Syndrome Toxin-1 Alters Human Aortic Endothelial Cell Function
Authors: K Kulhankova, KJ Kinney, JM Stach, FA Gourronc, IM Grumbach, AJ Klingelhut, W Salgado-Pa
Infect. Immun., 2018-02-20;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
A Three-Dimensional Cell Culture System To Model RNA Virus Infections at the Blood-Brain Barrier
Authors: JC Bramley, CG Drummond, NJ Lennemann, CA Good, KS Kim, CB Coyne
mSphere, 2017-06-21;2(3):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Synergic effects of VEGF-A and SDF-1 on the angiogenic properties of endothelial progenitor cells
Authors: Gabriela Odent Grig
J Tissue Eng Regen Med, 2016-12-12;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Targeting Syndecan-1, a molecule implicated in the process of vasculogenic mimicry, enhances the therapeutic efficacy of the L19-IL2 immunocytokine in human melanoma xenografts
Authors: Paola Orecchia, Romana Conte, Enrica Balza, Gabriella Pietra, Maria Cristina Mingari, Barbara Carnemolla
Oncotarget
-
Abluminal Stimulation of Sphingosine 1-Phosphate Receptors 1 and 3 Promotes and Stabilizes Endothelial Sprout Formation
Authors: Anusuya Das, Steven M. Lenz, Anthony O. Awojoodu, Edward A. Botchwey
Tissue Engineering Part A
-
Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs.
Authors: Lindsley RC, Gill JG, Murphy TL, Langer EM, Cai M, Mashayekhi M, Wang W, Niwa N, Nerbonne JM, Kyba M, Murphy KM
Cell Stem Cell, 2008-07-03;3(1):55-68.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption.
Authors: Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Drel VR, Obrosova IG, Pacher P
Am. J. Physiol. Heart Circ. Physiol., 2007-03-23;293(1):H610-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC
FAQs
-
Why does the staining protocol with this Cadherin antibody use buffers containing Ca2+ and Mg2+?
The staining protocol with this and other Cadherin antibodies uses buffer containing Ca2+ and Mg2+ because Cadherin function is Calcium-dependent.
Reviews for Human VE-Cadherin Antibody
Average Rating: 4.5 (Based on 2 Reviews)
Have you used Human VE-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: