Human/Mouse/Rat Vimentin Antibody Summary
Ser2-Glu466
Accession # P08670
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Vimentin in Human Colon via Multiplex Immunofluorescence staining on COMET™ Vimentin was detected in immersion fixed paraffin-embedded sections of human colon using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 1 µg/mL at 37 ° Celsius for 4 minutes. Before incubation with the primary antibody, tissue underwent an all-in-one dewaxing and antigen retrieval preprocessing using PreTreatment Module (PT Module) and Dewax and HIER Buffer H (pH 9). Tissue was stained using the Alexa Fluor™ Plus 647 Goat anti-Rat IgG Secondary Antibody at 1:200 at 37 ° Celsius for 2 minutes. (Yellow; Lunaphore Catalog # DR647RT) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the cytoplasm and cytoskeleton. Protocol available in COMET™ Panel Builder.
 View Larger
View Larger
Detection of Vimentin in Human Liver via seqIF™ staining on COMET™ Vimentin was detected in immersion fixed paraffin-embedded sections of human liver using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 1 µg/mL at 37 ° Celsius for 4 minutes. Before incubation with the primary antibody, tissue underwent an all-in-one dewaxing and antigen retrieval preprocessing using PreTreatment Module (PT Module) and Dewax and HIER Buffer H (pH 9; Epredia Catalog # TA-999-DHBH). Tissue was stained using the Alexa Fluor™ 647 Goat anti-Rat IgG Secondary Antibody at 1:200 at 37 ° Celsius for 2 minutes. (Yellow; Lunaphore Catalog # DR647RT) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the cytoplasm and cytoskeleton. Protocol available in COMET™ Panel Builder.
 View Larger
View Larger
Detection of Vimentin in Human Kidney via seqIF™ staining on COMET™ Vimentin was detected in immersion fixed paraffin-embedded sections of human kidney using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 1 µg/mL at 37 ° Celsius for 4 minutes. Before incubation with the primary antibody, tissue underwent an all-in-one dewaxing and antigen retrieval preprocessing using PreTreatment Module (PT Module) and Dewax and HIER Buffer H (pH 9; Epredia Catalog # TA-999-DHBH). Tissue was stained using the Alexa Fluor™ 647 Goat anti-Rat IgG Secondary Antibody at 1:200 at 37 ° Celsius for 2 minutes. (Yellow; Lunaphore Catalog # DR647RT) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the cytoplasm and cytoskeleton. Protocol available in COMET™ Panel Builder.
 View Larger
View Larger
Detection of Vimentin in Mouse Kidney via seqIF™ staining on COMET™ Vimentin Antibody was detected in immersion fixed paraffin-embedded sections of Mouse Kidney using Rat Anti-Mouse Vimentin, Monoclonal Antibody (Catalog # MAB2105) at 10ug/mL at 37 ° Celsius for 4 minutes. Before incubation with the primary antibody, tissue underwent an all-in-one dewaxing and antigen retrieval preprocessing using PreTreatment Module (PT Module) and Dewax and HIER Buffer H (pH 9; Epredia Catalog # TA-999-DHBH). Tissue was stained using the Alexa Fluor™ 647 Goat anti-Rat IgG Secondary Antibody at 1:200 at 37 ° Celsius for 2 minutes. (Yellow; Lunaphore Catalog # DR647RT) and counterstained with DAPI (blue; Lunaphore Catalog # DR100). Specific staining was localized to the membrane. Protocol available in COMET™ Panel Builder.
 View Larger
View Larger
Detection of Human Vimentin by Western Blot. Western blot shows lysates of Jurkat human acute T cell leukemia cell line and K562 human chronic myelogenous leukemia cell line. PVDF membrane was probed with 2 µg/mL of Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) followed by HRP-conjugated Anti-Rat IgG Secondary Antibody (Catalog # HAF005). A specific band was detected for Vimentin at approximately 55 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
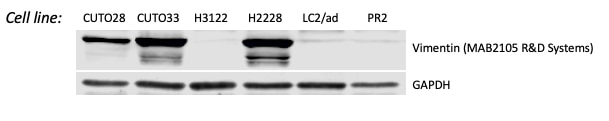
Detection of Mouse and Rat Vimentin by Western Blot. Western blot shows lysates of MEF mouse embryonic feeder cells, NIH-3T3 mouse embryonic fibroblast cell line, Rat-2 rat embryonic fibroblast cell line, and NR8383 rat alveolar macrophage cell line. PVDF membrane was probed with 1 µg/mL of Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) followed by HRP-conjugated Anti-Rat IgG Secondary Antibody (Catalog # HAF005). A specific band was detected for Vimentin at approximately 55 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Vimentin in NTera‑2 Human Cell Line. Vimentin was detected in immersion fixed NTera-2 human testicular embryonic carcinoma cell line using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (yellow; Catalog # NL013) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Vimentin in A549 Human Cell Line. Vimentin was detected in immersion fixed A549 human lung carcinoma cell line using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 493-conjugated Anti-Rat IgG Secondary Antibody (green; Catalog # NL015) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Vimentin in Mouse Cortical Stem Cells. Vimentin was detected in immersion fixed mouse cortical stem cells using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (red; Catalog # NL013) and counterstained with DAPI (blue). Specific staining was localized to cytoskeleton. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Vimentin in Rat Cortical Stem Cells. Vimentin was detected in immersion fixed rat cortical stem cells using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (red; Catalog # NL013) and counterstained with DAPI (blue). Specific staining was localized to cytoskeleton. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Vimentin in Human Tonsil. Vimentin was detected in immersion fixed paraffin-embedded sections of human tonsil using Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) at 0.5 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC005). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cytoplasm. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Vimentin in A172 Human Cell Line by Flow Cytometry. A172 human glioblastoma cell line was stained with Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105, filled histogram) or isotype control antibody (Catalog # MAB006, open histogram) followed by anti-Rat IgG PE-conjugated secondary antibody (Catalog # F0105B). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Detection of Human Vimentin by Simple WesternTM. Simple Western lane view shows lysates of Jurkat human acute T cell leukemia cell line, loaded at 0.2 mg/mL. A specific band was detected for Vimentin at approximately 58 kDa (as indicated) using 10 µg/mL of Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) followed by 1:50 dilution of HRP-conjugated Anti-Rat IgG Secondary Antibody (Catalog # HAF005). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Western Blot Shows Human Vimentin Specificity by Using Knockout Cell Line. Western blot shows lysates of K562 human chronic myelogenous leukemia parental cell line and Vimentin knockout K562 cell line (KO). PVDF membrane was probed with 2 µg/mL of Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (Catalog # MAB2105) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). A specific band was detected for Vimentin at approximately 55 kDa (as indicated) in the parental K562 cell line, but is not detectable in knockout K562 cell line. GAPDH (MAB5718) is shown as a loading control. This experiment was conducted under reducing conditions and using Western Blot Buffer Group 1.
 View Larger
View Larger
Detection of Human Vimentin by Immunocytochemistry/Immunofluorescence A very minimal population of human vimentin+ HSCs/myofibroblasts express a primary cilium, with none detected on CD31+ endothelial cells.Human ALD liver tissue was examined for the expression of primary cilia ( alpha -acetylated tubulin, green; gamma -tubulin, red) by vimentin+ (grey) HSCs/myofibroblasts (A) or CD31+ (grey) ECs (C). (A) The majority of vimentin+ cells were Pc-ve in the tissues examined. Representative image shown, displaying absence of Pc on vimentin+ cells. To confirm this result, ciliary protein Arl13b (green) was co-stained with vimentin (grey). Rare Arl13b ciliary structures (arrow) co-localised with vimentin+ cells. Final panel in A illustrates rare Pc+ ( alpha -acetylated tubulin, green; gamma -tubulin, red) vimentin+ (grey) HSCs/myofibroblasts, at the cirrhotic interface. (B) Number of vimentin+ Pc+ cells or vimentin+ Pcneg cells per FOV (n = 3 ALD samples, 8 FOV/sample). (C) No Pc were detected on CD31+ cells in the tissues examined (ALD n = 3, 8 FOV/sample). Representative image shown. All images obtained using confocal microscopy, 63x objective. DAPI, blue. White arrows illustrate Pc. * Non-specific liver autofluorescence. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0171480), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Vimentin by Immunocytochemistry/Immunofluorescence Widespread GLI expression in human donor and cirrhotic liver.(A) Frozen (4 μm) human donor (n = 5), and cirrhotic liver sections [ALD (n = 6), NASH (n = 3), PBC (n = 1)] were screened for GLI2 (red) expression by immunofluorescence. Representative images taken at 5x or 40x (insets) objective shown. DAPI, blue. (B) qRT-PCR for GLI1 and GLI3 transcript in human donor or ALD samples. Mean±S.E.M. Significant (*) difference between means (One-sided student t-test, **p<0.005). Western blot for full-length GLI1 protein (>150 kDa) in donor (Don) or ALD patient samples. Densitometry analysis with GLI1 normalised to GAPDH (Image J). Mean±S.E.M; **p = 0.0093 (Two-sided student t-test). (C) Nuclear GLI2 (green) expression in EpCAM+ (red) LPCs in donor, ALD, PBC and NASH liver. (D) Nuclear GLI2 (green) expression demonstrated within CD31+ (red) ECs, CK18+ (red) hepatocytes, CD45+ (red) leukocytes and vimentin+ (red) HSCs/myofibroblasts, in ALD. 63x objective. (E) Maximum intensity projection illustrating close physical association between EpCAM+ LPCs (green) and vimentin+ HSCs/myofibroblasts (red), both of which express GLI2 (grey), in ALD tissue. Arrows indicate myofibroblasts directly contacting LPCs. Confocal microscopy, 63x objective. Quantitation (%) of EpCAM+ GLI2+ cells and vimentin+ GLI2+ cells within the same FOV (n = 3 ALD samples, 8 FOV/sample). Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0171480), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Vimentin
Vimentin is a 57 kDa class III intermediate filament (IF) protein that belongs to the intermediate filament family. It is the predominant IF in cells of mesenchymal origin such as vascular endothelium and blood cells (1-3). The human Vimentin cDNA encodes a 466 amino acid (aa) protein that contains head and tail regions with multiple regulatory Ser/Thr phosphorylation sites, and a central rod domain with three coiled-coil regions separated by linkers (1, 2). Human Vimentin shares 97-98% aa identity with mouse, rat, ovine, bovine, and canine Vimentin. Sixteen Vimentin coiled-coil dimers self-assemble to form intermediate (10-12 nm wide) filaments (4). These filaments then anneal longitudinally to form non-polarized fibers that support cell structure and withstand stress (4). IF fibers are highly dynamic, and half-life depends on the balance between kinase and phosphatase activity. For example, phosphorylation followed by dephosphorylation drives IF disintegration, followed by reorganization during mitosis (1, 5, 6). Interactions of head and tail domains link IFs with other structures such as actin and microtubule cytoskeletons (7). Vimentin is involved in positioning autophagosomes, lysosomes and the Golgi complex within the cell (8). It facilitates cell migration and motility by recycling internalized trailing edge integrins back to the cell surface at the leading edge (9-11). Vimentin helps maintain the lipid composition of cellular membranes, and caspase cleavage of Vimentin is a key event in apoptosis (8, 12). Phosphorylation promotes secretion of Vimentin by TNF-alpha -stimulated macrophages (13). Extracellular Vimentin has been shown to associate with several microbes, and appears to promote an antimicrobial oxidative burst (13, 14). Cell-associated Vimentin can also interact with NKp46 to recruit NK cells to tuberculosis-infected monocytes (15).
- Omary, M.B. et al. (2006) Trends Biochem. Sci. 31:383.
- Ivaska, J. et al. (2007) Exp. Cell Res. 313:2050.
- Ferrari, S. et al. (1986) Mol. Cell. Biol. 6:3614.
- Sokolova, A.V. et al. (2006) Proc. Natl. Acad. Sci. USA 103:16206.
- Eriksson, J.E. et al. (2004) J. Cell Sci. 117:919.
- Li, Q-F. et al. (2006) J. Biol. Chem. 281:34716.
- Esue, O. et al. (2006) J. Biol. Chem. 281:30393.
- Styers, M.L. et al. (2005) Traffic 6:359.
- McInroy, L. and A. Maata (2007) Biochem. Biophys. Res. Commun. 360:109.
- Nieminen, M. et al. (2006) Nat. Cell Biol. 8:156.
- Ivaska, J. et al. (2005) EMBO J. 24:3834.
- Byun, Y. et al. (2001) Cell Death Differ. 8:443.
- Mor-Vaknin, N. et al. (2003) Nat. Cell Biol. 5:59.
- Zou, Y. et al. (2006) Biochem. Biophys. Res. Commun. 351:625.
- Garg, A. et al. (2006) J. Immunol. 177:6192.
Product Datasheets
Citations for Human/Mouse/Rat Vimentin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
44
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
CAMSAPs organize an acentrosomal microtubule network from basal varicosities in radial glial cells
Authors: Coquand L, Victoria GS, Tata A et al.
The Journal of cell biology
-
Estrogen enhances the bone regeneration potential of periodontal ligament stem cells derived from osteoporotic rats and seeded on nano-hydroxyapatite/collagen/poly(L-lactide).
Authors: E L, Xu W, Feng L, Liu Y, Cai D, Wen N, Zheng W
Int J Mol Med, 2016-04-12;37(6):1475-86.
-
Efficient and reproducible generation of human iPSC-derived cardiomyocytes and cardiac organoids in stirred suspension systems
Authors: Prondzynski, M;Berkson, P;Trembley, MA;Tharani, Y;Shani, K;Bortolin, RH;Sweat, ME;Mayourian, J;Yucel, D;Cordoves, AM;Gabbin, B;Hou, C;Anyanwu, NJ;Nawar, F;Cotton, J;Milosh, J;Walker, D;Zhang, Y;Lu, F;Liu, X;Parker, KK;Bezzerides, VJ;Pu, WT;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Characterization of primary human leptomeningeal cells in 2D culture
Authors: Abubaker, M;Greaney, A;Newport, D;Mulvihill, JJE;
Heliyon
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Single-cell transcriptomics of human cholesteatoma identifies an activin A-producing osteoclastogenic fibroblast subset inducing bone destruction
Authors: Shimizu, K;Kikuta, J;Ohta, Y;Uchida, Y;Miyamoto, Y;Morimoto, A;Yari, S;Sato, T;Kamakura, T;Oshima, K;Imai, R;Liu, YC;Okuzaki, D;Hara, T;Motooka, D;Emoto, N;Inohara, H;Ishii, M;
Nature communications
Species: Human
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Regulation of cellular contractile force, shape and migration of fibroblasts by oncogenes and Histone deacetylase 6
Authors: Ana López-Guajardo, Azeer Zafar, Khairat Al Hennawi, Valentina Rossi, Abdulaziz Alrwaili, Jessica D. Medcalf et al.
Frontiers in Molecular Biosciences
-
Transplantation of adipose tissue-derived microvascular fragments promotes therapy of critical limb ischemia
Authors: Park, GT;Lim, JK;Choi, EB;Lim, MJ;Yun, BY;Kim, DK;Yoon, JW;Hong, YG;Chang, JH;Bae, SH;Ahn, JY;Kim, JH;
Biomaterials research
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
5-Azacytidine- and retinoic-acid-induced reprogramming of DCCs into dormancy suppresses metastasis via restored TGF-?-SMAD4 signaling
Authors: Singh, DK;Carcamo, S;Farias, EF;Hasson, D;Zheng, W;Sun, D;Huang, X;Cheung, J;Nobre, AR;Kale, N;Sosa, MS;Bernstein, E;Aguirre-Ghiso, JA;
Cell reports
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Corneal epithelial basement membrane assembly is mediated by epithelial cells in coordination with corneal fibroblasts during wound healing
Authors: Shiju TM, Sampaio LP, Hilgert GSL, Wilson SE
Molecular vision
-
Efficient Isolation and Expansion of Limbal Melanocytes for Tissue Engineering
Authors: Polisetti, N;Reinhard, T;Schlunck, G;
International journal of molecular sciences
Species: Human
Sample Types: Whole Cells
Applications: ICC -
ALD-R491 regulates vimentin filament stability and solubility, cell contractile force, cell migration speed and directionality
Authors: Hyejeong Rosemary Kim, Samantha J. Warrington, Ana López-Guajardo, Khairat Al Hennawi, Sarah L. Cook, Zak D. J. Griffith et al.
Frontiers in Cell and Developmental Biology
-
T cell deficiency precipitates antibody evasion and emergence of neurovirulent polyomavirus
Authors: MD Lauver, G Jin, KN Ayers, SN Carey, CS Specht, CS Abendroth, AE Lukacher
Elife, 2022-11-07;11(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lesion environments direct transplanted neural progenitors towards a wound repair astroglial phenotype in mice
Authors: O'Shea TM, Ao Y, Wang S et al.
Nature Communications
-
Engineering of immune checkpoints B7-H3 and CD155 enhances immune compatibility of MHC-I-/- iPSCs for beta cell replacement
Authors: R Chimienti, T Baccega, S Torchio, F Manenti, S Pellegrini, A Cospito, A Amabile, MT Lombardo, P Monti, V Sordi, A Lombardo, M Malnati, L Piemonti
Cell Reports, 2022-09-27;40(13):111423.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
NR2F1 is a barrier to dissemination of early stage breast cancer cells
Authors: C Rodriguez-, N Kale, MJ Carlini, N Shrivastav, AA Rodrigues, B Khalil, JJ Bravo-Cord, M Alexander, J Ji, Y Hong, F Behbod, MS Sosa
Cancer Research, 2022-06-15;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: IF -
Suspension culture promotes serosal mesothelial development in human intestinal organoids
Authors: MM Capeling, S Huang, CJ Childs, JH Wu, YH Tsai, A Wu, N Garg, EM Holloway, N Sundaram, C Bouffi, M Helmrath, JR Spence
Cell Reports, 2022-02-15;38(7):110379.
Species: Human
Sample Types: Organoids
Applications: IHC -
The LRRK2 G2019S mutation alters astrocyte-to-neuron communication via extracellular vesicles and induces neuron atrophy in a human iPSC-derived model of Parkinson's disease.
Authors: de Rus Jacquet A, Tancredi J, Lemire A, DeSantis M, Li W, O'Shea E
Elife, 2021-09-30;10(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Nrf1 promotes heart regeneration and repair by regulating proteostasis and redox balance
Authors: M Cui, A Atmanli, MG Morales, W Tan, K Chen, X Xiao, L Xu, N Liu, R Bassel-Dub, EN Olson
Nature Communications, 2021-09-06;12(1):5270.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Echinochrome A Treatment Alleviates Fibrosis and Inflammation in Bleomycin-Induced Scleroderma
Authors: GT Park, JW Yoon, SB Yoo, YC Song, P Song, HK Kim, J Han, SJ Bae, KT Ha, NP Mishchenko, SA Fedoreyev, VA Stonik, MB Kim, JH Kim
Marine Drugs, 2021-04-23;19(5):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Primary cilia-dependent lipid raft/caveolin dynamics regulate adipogenesis
Authors: D Yamakawa, D Katoh, K Kasahara, T Shiromizu, M Matsuyama, C Matsuda, Y Maeno, M Watanabe, Y Nishimura, M Inagaki
Cell Reports, 2021-03-09;34(10):108817.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Heterogeneous Manifestations of Epithelial-Mesenchymal Plasticity of Circulating Tumor Cells in Breast Cancer Patients
Authors: LA Tashireva, OE Savelieva, ES Grigoryeva, YV Nikitin, EV Denisov, SV Vtorushin, MV Zavyalova, NV Cherdyntse, VM Perelmuter
International Journal of Molecular Sciences, 2021-03-02;22(5):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity
Authors: SK Sarvestani, S Signs, B Hu, Y Yeu, H Feng, Y Ni, DR Hill, RC Fisher, S Ferrandon, RK DeHaan, J Stiene, M Cruise, TH Hwang, X Shen, JR Spence, EH Huang
Nature Communications, 2021-01-11;12(1):262.
Species: Human
Sample Types: Organoid
Applications: IHC -
miR-146b Functions as an Oncogene in Oral Squamous Cell Carcinoma by Targeting HBP1
Authors: Kui Li, Zheng Zhou, Ju Li, Rui Xiang
Technol Cancer Res Treat
-
Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution
Authors: Z Wang, M Cui, AM Shah, W Tan, N Liu, R Bassel-Dub, EN Olson
Cell Reports, 2020-12-08;33(10):108472.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Quantitative proteomic comparison of myofibroblasts derived from bone marrow and cornea
Authors: P Saikia, JS Crabb, LL Dibbin, MJ Juszczak, B Willard, GF Jang, TM Shiju, JW Crabb, SE Wilson
Sci Rep, 2020-10-07;10(1):16717.
Species: Rabbit
Sample Types: Whole Cells
Applications: ICC -
Antibody escape by polyomavirus capsid mutation facilitates neurovirulence
Authors: Matthew D Lauver, Daniel J Goetschius, Colleen S Netherby-Winslow, Katelyn N Ayers, Ge Jin, Daniel G Haas et al.
eLife
-
Dualism of FGF and TGF-beta Signaling in Heterogeneous Cancer-Associated Fibroblast Activation with ETV1 as a Critical Determinant
Authors: Pino Bordignon, Giulia Bottoni, Xiaoying Xu, Alma S. Popescu, Zinnia Truan, Emmanuella Guenova et al.
Cell Reports
-
Sensitive and easy screening for circulating tumor cells by flow cytometry
Authors: A Lopresti, F Malergue, F Bertucci, ML Liberatosc, S Garnier, Q DaCosta, P Finetti, M Gilabert, JL Raoul, D Birnbaum, C Acquaviva, E Mamessier
JCI Insight, 2019-06-13;5(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors
Authors: Zsuzsanna Szvicsek, Ádám Oszvald, Lili Szabó, Gyöngyvér Orsolya Sándor, Andrea Kelemen, András Áron Soós et al.
Cellular and Molecular Life Sciences
-
Desmin deficiency is not sufficient to prevent corneal fibrosis
Authors: Alexandra Pietraszkiewicz, Christopher Hampton, Sonny Caplash, Ling Lei, Yassemi Capetanaki, Gauri Tadvalkar et al.
Experimental Eye Research
-
Platelet glycoprotein VI and C-type lectin-like receptor 2 deficiency accelerates wound healing by impairing vascular integrity in mice
Authors: S Wichaiyo, S Lax, SJ Montague, Z Li, B Grygielska, JA Pike, EJ Haining, A Brill, SP Watson, J Rayes
Haematologica, 2019-02-07;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
RNA sequencing reveals upregulation of a transcriptomic program associated with stemness in metastatic prostate cancer cells selected for taxane resistance
Authors: CK Cajigas-Du, SR Martinez, L Woods-Burn, AM Durán, S Roy, A Basu, JA Ramirez, GL Ortiz-Hern, L Ríos-Colón, E Chirshev, ES Sanchez-He, U Soto, C Greco, C Boucheix, X Chen, J Unternaehr, C Wang, CA Casiano
Oncotarget, 2018-07-13;9(54):30363-30384.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Correlation of High-Risk Soft Tissue Sarcoma Biomarker Expression Patterns with Outcome following Neoadjuvant Chemoradiation
Authors: John M. Kane, Anthony Magliocco, Qiang Zhang, Dian Wang, Alex Klimowicz, Jonathan Harris et al.
Sarcoma
-
Acute Drug Effects on the Human Placental Tissue: The Development of a Placental Murine Xenograft Model
Authors: M Verheecke, E Hermans, S Tuyaerts, E Souche, R Van Bree, G Verbist, T Everaert, J Van Houdt, K Van Calste, F Amant
Reprod Sci, 2018-02-13;0(0):1933719118756.
Species: Xenograft
Sample Types: Whole Cells
Applications: Flow Cytometry -
MicroRNA-141 inhibits epithelial-mesenchymal transition, and ovarian cancer cell migration and invasion
Authors: Qinghua Ye, Lei Lei, Lingyun Shao, Jing Shi, Jun Jia, Xiaowen Tong
Molecular Medicine Reports
-
Simultaneous Isolation of Three Different Stem Cell Populations from Murine Skin
Authors: Maria Fernanda Forni, Aline Ramos Maia Lobba, Alexandre Hamilton Pereira Ferreira, Mari Cleide Sogayar
PLOS ONE
-
Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation.
Authors: Procopio M, Laszlo C, Al Labban D, Kim D, Bordignon P, Jo S, Goruppi S, Menietti E, Ostano P, Ala U, Provero P, Hoetzenecker W, Neel V, Kilarski W, Swartz M, Brisken C, Lefort K, Dotto G
Nat Cell Biol, 2015-08-24;17(9):1193-204.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
The TNF Family Molecules LIGHT and Lymphotoxin alphabeta Induce a Distinct Steroid-Resistant Inflammatory Phenotype in Human Lung Epithelial Cells.
Authors: da Silva Antunes R, Madge L, Soroosh P, Tocker J, Croft M
J Immunol, 2015-07-24;195(5):2429-41.
-
GLI1, CTNNB1 and NOTCH1 protein expression in a thymic epithelial malignancy tissue microarray.
Authors: Riess J, West R, Dean M, Klimowicz A, Neal J, Hoang C, Wakelee H
2015-02-01;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Evaluation of E-cadherin, beta -catenin and vimentin protein expression using quantitative immunohistochemistry in nasopharyngeal carcinoma patients
Authors: Desirée Hao, Tien Phan, Amanda Jagdis, Jodi E Siever, Alexander C Klimowicz, Janessa J Laskin et al.
Clinical & Investigative Medicine
-
Effects of Wnt5a protein on proliferation and apoptosis in JAR choriocarcinoma cells
Authors: Sha Peng, Junlin Zhang, Jiahuan Chen, Huayan Wang
Molecular Medicine Reports
-
Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures.
Authors: Vrana NE, Builles N, Justin V, Bednarz J, Pellegrini G, Ferrari B, Damour O, Hulmes DJ, Hasirci V
Invest. Ophthalmol. Vis. Sci., 2008-08-15;49(12):5325-31.
Species: Human
Sample Types: Whole Tissue
Applications: IHC-P -
An IRAK1-PIN1 signalling axis drives intrinsic tumour resistance to radiation therapy
Authors: Liu PH, Shah RB, Li Y et al.
Nat. Cell Biol.
-
Modeling Tumor Phenotypes In�Vitro with Three-Dimensional Bioprinting
Authors: EM Langer, BL Allen-Pete, SM King, ND Kendsersky, MA Turnidge, GM Kuziel, R Riggers, R Samatham, TS Amery, SL Jacques, BC Sheppard, JE Korkola, JL Muschler, G Thibault, YH Chang, JW Gray, SC Presnell, DG Nguyen, RC Sears
Cell Rep, 2019-01-15;26(3):608-623.e6.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse/Rat Vimentin Antibody
Average Rating: 4.5 (Based on 6 Reviews)
Have you used Human/Mouse/Rat Vimentin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
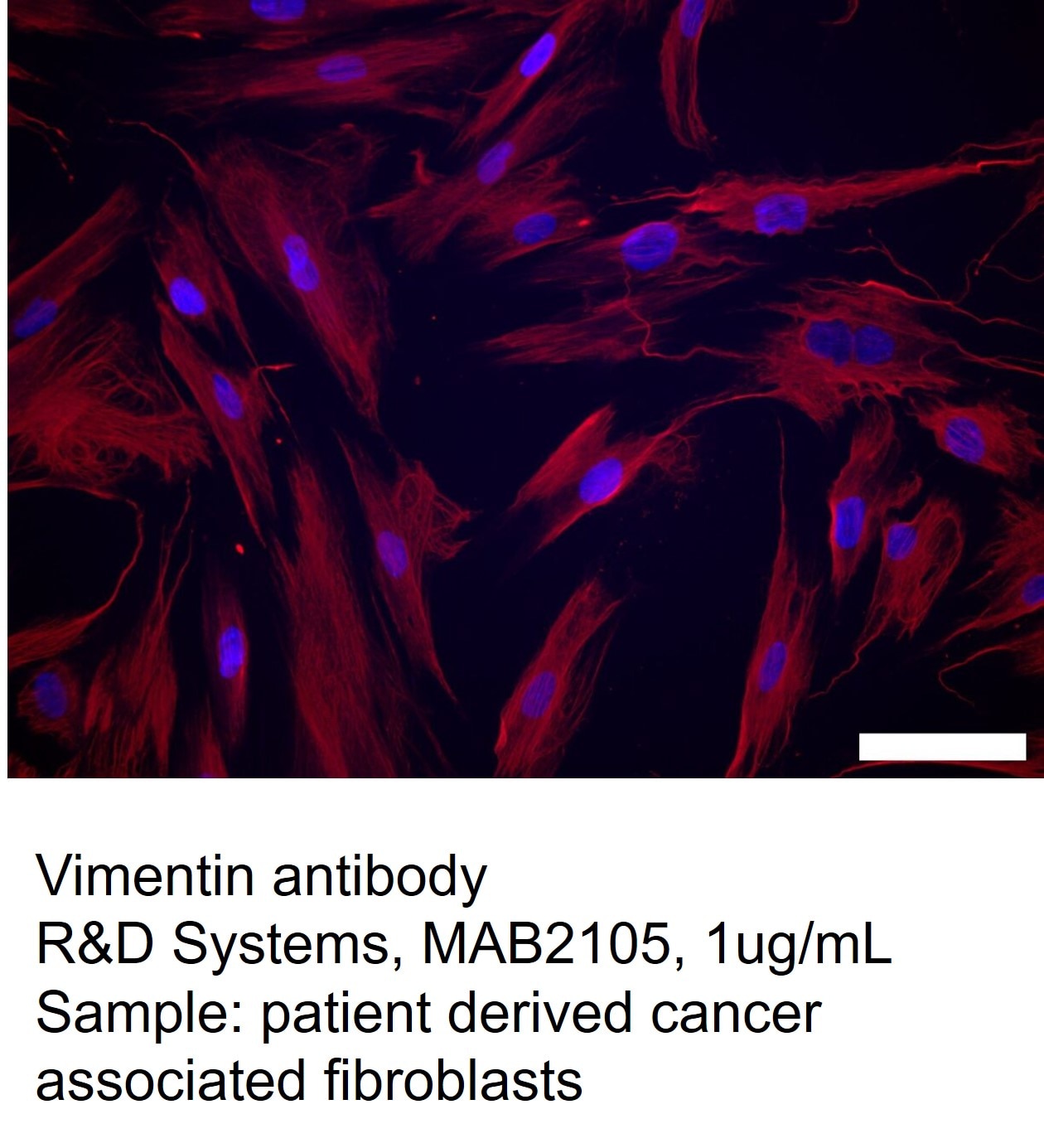
Human cancer associated fibroblast cells were stained with Vimentin antibody to confirm identity.
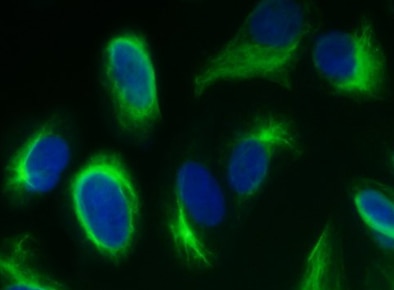
Vimentin expression in human fibroblasts in culture. Vimentin (green) was used as a fibroblast marker in miofibroblast differentiation assays (a-SMA in red, as a miofibroblast marker).