Human DC-SIGN+DC-SIGNR Antibody Summary
Accession # Q9H2X3
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of DC‑SIGN+DC‑SIGNR in Human DC‑SIGN or DC-SIGNR Transfected 3T3 Mouse Cell Line by Flow Cytometry. Human DC-SIGN and DC-SIGNR transfected 3T3 mouse embryonic fibroblast cell line were stained with Mouse Anti-Human DC-SIGN+ DC-SIGNR Monoclonal Antibody (Catalog # MAB1621, filled histograms) or isotype control antibody (Catalog # MAB003, open histogram), followed by Phycoerythrin-conjugated Anti-Mouse IgG F(ab')2Secondary Antibody (Catalog # F0102B).
 View Larger
View Larger
Detection of DC‑SIGN+DC‑SIGNR in Human Monocyte Derived Dendritic Cells by Flow Cytometry. Human monocyte derived dendritic cells were stained with Mouse Anti-Human DC-SIGN+ DC-SIGNR Monoclonal Antibody (Catalog # MAB1621), followed by PE-conjugated anti-mouse secondary antibody (Catalog # F0102B) and Human B7-2/CD86 Fluorescein-conjugated Monoclonal Antibody (Catalog # FAB141F).Quadrant markers were set based on isotype control antibody staining (Catalog # MAB003).
 View Larger
View Larger
DC‑SIGN+DC‑SIGNR in Human Lymphoma. DC-SIGN+DC-SIGNR was detected in immersion fixed paraffin-embedded sections of human lymphoma using 25 µg/mL Mouse Anti-Human DC-SIGN+ DC-SIGNR Monoclonal Antibody (Catalog # MAB1621) overnight at 4 °C. Tissue was stained with the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (brown; Catalog # CTS002) and counter-stained with hematoxylin (blue). View our protocol for Chromogenic IHC Staining of Paraffin-embedded Tissue Sections.
 View Larger
View Larger
Detection of Human DC-SIGN+DC-SIGNR by Block/Neutralize The C type lectin DC-SIGN enhances infection of human cells by tick cell-derived rUUKV S23. (A) BHK-21 cells were infected (at an MOI of 0.1) with rUUKV S23 derived from IRE/CTVM19 cells for 18 h and immunostained for N, GN, and GC proteins prior to flow cytometry analysis. (B) Parental (Raji) and DC-SIGN-expressing Raji cells (Raji DC-SIGN+) were infected with IRE/CTVM19 cell-derived rUUKV S23 and analyzed by flow cytometry 16 h after immunostaining against the viral nucleoprotein. (C) Parental (HeLa) and DC-SIGN-expressing HeLa cells (HeLa DC-SIGN+) were exposed to various MOIs of IRE/CTVM19 cell-derived rUUKV S23. The next day, infected cells were immunostained for the intracellular virus nucleoprotein N using the anti-N primary mouse monoclonal antibody 8B11A3 and an AF488-coupled anti-mouse secondary monoclonal antibody (green). Nuclei were stained with Hoechst (blue), and samples were analyzed by wide-field microscopy. (D) Raji DC-SIGN-expressing cells were exposed to IRE/CTVM19 cell-derived rUUKV S23 (MOI of ∼1) in the presence of inhibitors blocking DC-SIGN, namely, EDTA (5 mM) or the neutralizing mouse monoclonal antibody mAb1621 (25 μg · ml−1). Intracellular viral antigens were detected by immunostaining with an anti-UUKV rabbit polyclonal antibody, followed by incubation with AF647-conjugated secondary antibodies. Infection was analyzed by flow cytometry 18 h later and normalized to infection of DC-SIGN-expressing Raji cells in the absence of inhibitor (as a percentage of the control). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/27194760), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: DC-SIGN+DC-SIGNR
DC-SIGN (Dendritic Cell- Specific ICAM-3 Grabbing Non-Integrin) has been shown to play an important role in regulating dendritic cell (DC) and T cell interactions, including antigen presentation to T cells and enhancement of transinfection of CD4+ T cells by HIV-1 (1, 2). Efforts to identify additional type II membrane proteins resulted in the isolation of a molecule related in sequence to DC-SIGN known as DC-SIGNR (DC-SIGN Related) (3, 4). DC-SIGNR shares 73 - 80% amino acid homology with DC-SIGN and is located on human chromosome 19p13.3. Its structure is similar to DC-SIGN and therefore binds mannose residues in a calcium dependent fashion, including ICAM-3 and HIV-1 gp120 (5). DC-SIGNR, also known as L-SIGN (Liver/Lymph node-Specific ICAM-3-Grabbing Non-integrin) and DC-SIGNR, is polymorphic since allelic variations of the exon 4 encoded sequence have been isolated (5). This is further supported by a study demonstrating the ability to isolate a large repertoire of DC-SIGNR transcripts largely the result of alternative splicing of the 7 coding exons (6). L-SIGN/DC-SIGNR is primarily transcribed in the liver and lymph nodes but not in monocyte derived DC (5). Expression of L-SIGN/DC-SIGNR is restricted to endothelial cells derived from liver sinusoids, lymph nodes sinuses and capillaries (7) although variable expression in placenta and some monocytic cell lines has also been reported, including both membrane and soluble isoforms of the protein (6). Expression of DC-SIGN is induced during the in-vitro generation of DC from either monocytes or bone marrow progenitors, with maximal surface expression at day 7 of culture (1). Immature DC in the skin and mature DC in the tonsil have been demonstrated to express DC-SIGN (8). Analysis of various tissues and cell lines suggests that DC-SIGN expression is restricted to DC (1) although a more recent report finds evidence of expression in placenta, resting monocytes and monocytic cell lines (6). This discrepancy may be partially related to the multiple isoforms of DC-SIGN transcripts, including both membrane and soluble forms, as well as exon splice variants reported in the latter study (6).
- Geijtenbeek, T.B.H. et al. (2000) Cell 100:575.
- Geijtenbeek, T.B.H. et al. (2000) Cell 100:587.
- Yokoyama-Kobayashi, M.T. et al. (1999) Gene 228:161.
- Soilleux, E.J. et al. (2000) J. Immunol. 165:2937.
- Bashirova, A.A. et al. (2001) J. Exp. Med. 193:671.
- Mummidi, S. et al. (2001) J. Biol. Chem. 276:33196..
- Pohlman, S. et al. (2001) Proc. Natl. Acad. Sci. USA 98:2670.
- Geijtenbeek, T.B.H. et al. (2000) Nature Immunol. 1:353.
Product Datasheets
Citations for Human DC-SIGN+DC-SIGNR Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
15
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
DCC regulates astroglial development essential for telencephalic morphogenesis and corpus callosum formation
Authors: Morcom L, Gobius I, Marsh AP et al.
eLife
-
Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study
Authors: Diop, MK;Molina, OE;Birlea, M;LaRue, H;Hovington, H;T�tu, B;Lacombe, L;Bergeron, A;Fradet, Y;Trudel, D;
Cancers
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Placental macrophages present distinct polarization pattern and effector functions depending on clinical onset of preeclampsia
Authors: Monika Horvat Mercnik, Carolin Schliefsteiner, Herbert Fluhr, Christian Wadsack
Frontiers in Immunology
-
Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell
Authors: M Puray-Chav, KM LaPak, TP Schrank, JL Elliott, DP Bhatt, MJ Agajanian, R Jasuja, DQ Lawson, K Davis, PW Rothlauf, Z Liu, H Jo, N Lee, K Tenneti, JE Eschbach, C Shema Mugi, EM Cousins, EW Cloer, HR Vuong, LA VanBlargan, AL Bailey, P Gilchuk, JE Crowe, MS Diamond, DN Hayes, SPJ Whelan, A Horani, SL Brody, D Goldfarb, MB Major, SB Kutluay
Cell Reports, 2021-06-23;0(0):109364.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
Failure of Decidualization and Maternal Immune Tolerance Underlies Uterovascular Resistance in Intra Uterine Growth Restriction
Authors: Caroline Dunk, Melissa Kwan, Aleah Hazan, Sierra Walker, Julie K. Wright, Lynda K. Harris et al.
Front Endocrinol (Lausanne)
-
Primary Human Placental Trophoblasts are Permissive for Zika Virus (ZIKV) Replication
Authors: KM Aagaard, A Lahon, MA Suter, RP Arya, MD Seferovic, MB Vogt, M Hu, F Stossi, MA Mancini, RA Harris, M Kahr, C Eppes, M Rac, MA Belfort, CS Park, D Lacorazza, R Rico-Hesse
Sci Rep, 2017-01-27;7(0):41389.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Uukuniemi virus as a tick-borne virus model
J Virol, 2016-07-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Characterization of Glycoprotein-Mediated Entry of Severe Fever with Thrombocytopenia Syndrome Virus
Authors: Hideki Tani, Masayuki Shimojima, Shuetsu Fukushi, Tomoki Yoshikawa, Aiko Fukuma, Satoshi Taniguchi et al.
Journal of Virology
-
Binding of HIV-1 gp120 to DC-SIGN promotes ASK-1-dependent activation-induced apoptosis of human dendritic cells.
Authors: Chen Y, Hwang S, Chan V, Chung N, Wang S, Li Z, Ma J, Lin C, Hsieh Y, Chang K, Kung S, Wu Y, Chu C, Tai H, Gao G, Zheng B, Yokoyama K, Austyn J, Lin C
PLoS Pathog, 2013-01-31;9(1):e1003100.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Hybrid, Silica-Coated, Janus-Like Plasmonic-Magnetic Nanoparticles
Authors: Georgios A. Sotiriou, Ann M. Hirt, Pierre-Yves Lozach, Alexandra Teleki, Frank Krumeich, Sotiris E. Pratsinis
Chemistry of Materials
-
Vaccine protection by live, attenuated simian immunodeficiency virus in the absence of high-titer antibody responses and high-frequency cellular immune responses measurable in the periphery.
Authors: Mansfield K, Lang SM, Gauduin MC, Sanford HB, Lifson JD, Johnson RP, Desrosiers RC
J. Virol., 2008-02-13;82(8):4135-48.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: ICC -
CCR5-, DC-SIGN-dependent endocytosis and delayed reverse transcription after human immunodeficiency virus type 1 infection in human astrocytes.
Authors: Deiva K, Khiati A, Hery C, Salim H, Leclerc P, Horellou P, Tardieu M
AIDS Res. Hum. Retroviruses, 2006-11-01;22(11):1152-61.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC, Neutralization -
L-SIGN (CD209L) isoforms differently mediate trans-infection of hepatoma cells by hepatitis C virus pseudoparticles.
Authors: Falkowska E, Durso R, Gardner J, Cormier E, Arrigale R, Ogawa R, Donovan G, Maddon P, Olson W, Dragic T
J Gen Virol, 2006-09-01;87(0):2571-6.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
SIV-associated myocarditis: viral and cellular correlates of inflammation severity.
Authors: Yearley JH, Pearson C, Carville A, Shannon RP, Mansfield KG
AIDS Res. Hum. Retroviruses, 2006-06-01;22(6):529-40.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC-P -
Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN.
Authors: Wu L, 107253, Bashirova AA, Martin TD, Villamide L, Mehlhop E, Chertov AO, Unutmaz D, Pope M, Carrington M, KewalRamani VN
IL-6 signalling biomarkers in hospitalised patients with moderate to severe SARS-CoV-2 infection in a single centre study in Sweden, 2002-01-29;99(3):1568-73.
Species: Human, Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Cells
Applications: Flow Cytometry
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human DC-SIGN+DC-SIGNR Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human DC-SIGN+DC-SIGNR Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
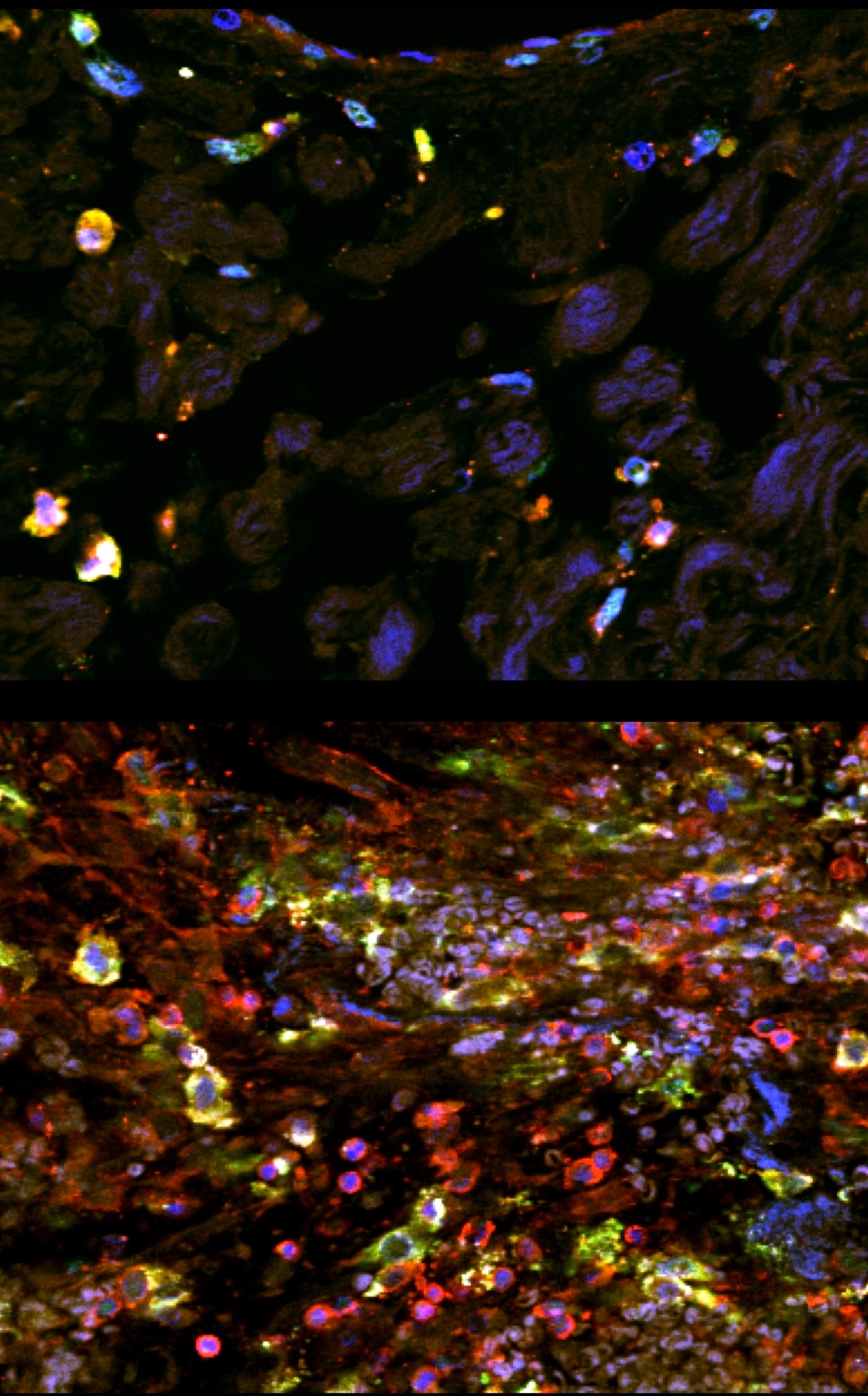
Immunostaining of human paraffin-embedded tissue showing DC-sign (green) costained with CD45 to label immune cells (red) and Dapi to label nuclei (blue). Tissue underwent antigen retrieval with Tris-EDTA (pH8) and DC-sign was used at 1:100. Top panel is from normal tissue, bottom panel is after injury.