Recombinant Human R-Spondin 1 Protein Summary
Product Specifications
Ser21-Ala263
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
4645-RS
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
4645-RS/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 100 μg/mL in PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Scientific Data
 View Larger
View Larger
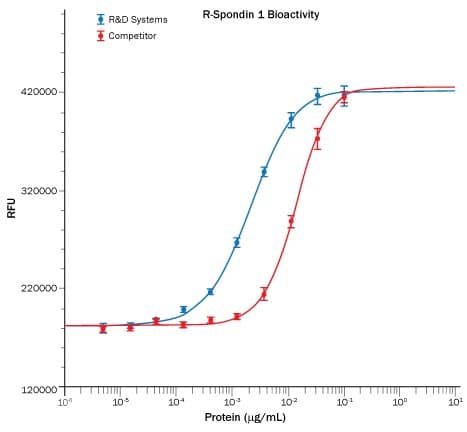
Recombinant Human R-Spondin 1 (Catalog # 4645-RS), in the presence of Recombinant Mouse Wnt-3a (Catalog # 1324-WN); 5 ng/mL), induces activation of beta-catenin in HEK293T cells measured using the Topflash assay (blue). The activity is approximately 7-fold greater than the competitor's R-Spondin 1 (red).
 View Larger
View Larger
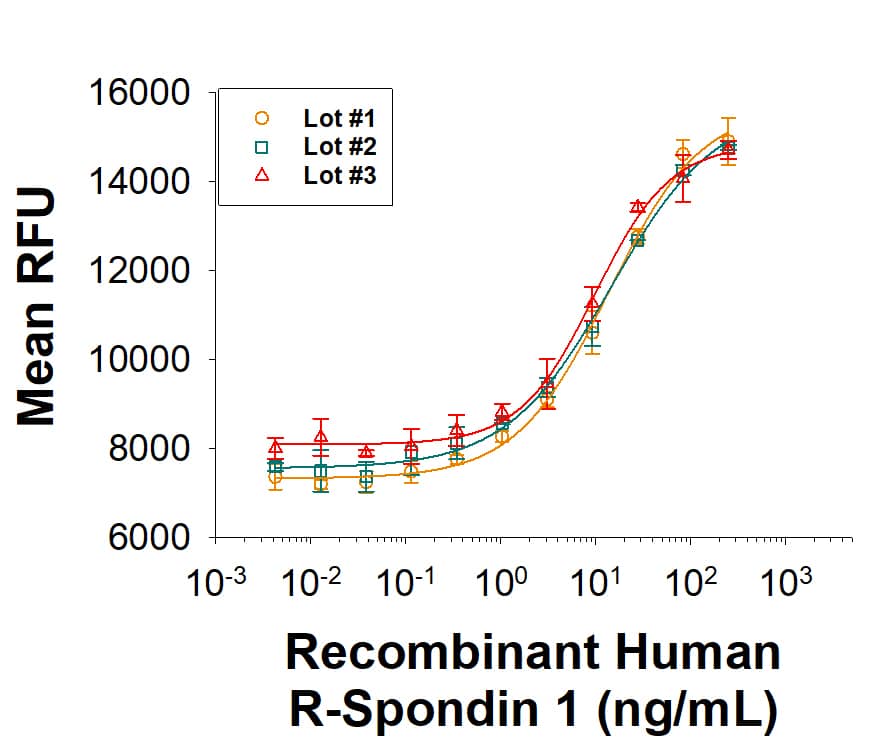
The lot-to-lot consistency of Recombinant Human R-Spondin 1 (Catalog # 4645-RS) was assessed by testing the ability of three independent lots of the protein to stimulate activation of beta-Catenin using a TOPflash beta-Catenin/TCF reporter assay in the HEK293T human kidney cell line, in the presence of 5 ng/mL Recombinant Mouse Wnt-3a (1324-WN). Each trace on the graph represents data obtained from Recombinant Human R-Spondin 1 from a different manufacturing run. The ED50 for this effect is 1.00-10.0 ng/mL in the presence of 5 ng/mL Recombinant Mouse Wnt-3a.
 View Larger
View Larger
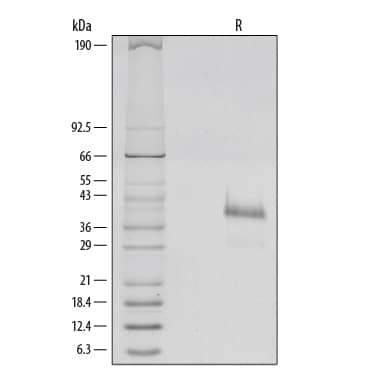
1 µg/lane of Recombinant Human R-Spondin 1 was resolved with SDS-PAGE and visualized by silver staining under reducing (R) conditions, showing a single band at 39 kDa.
 View Larger
View Larger
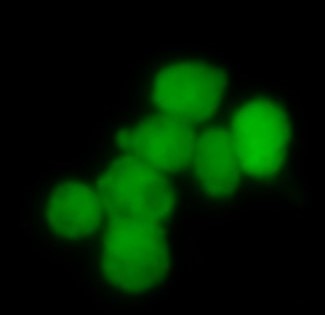
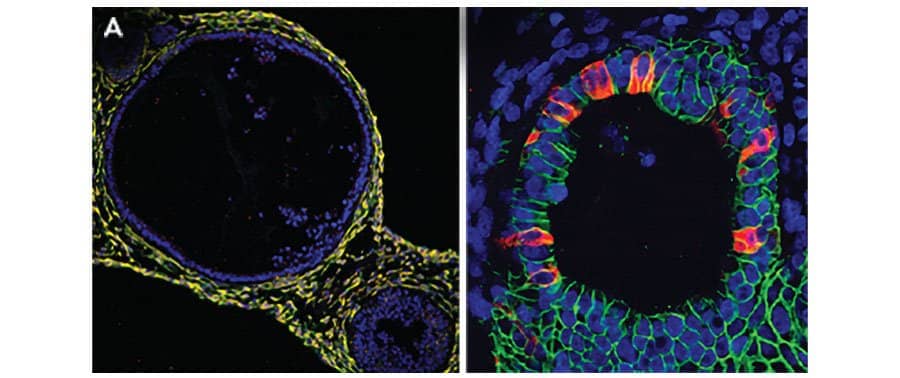
iPSC-derived human intestinal organoids were cultured using Cultrex™ UltiMatrix RGF Basement Membrane Extract (BME001-05) and intestinal organoid culture medium, which includes Recombinant Human R-Spondin 1 (Catalog # 4645-RS), Recombinant Human EGF (236-EG), Recombinant Human Noggin (6057-NG), and Recombinant Human Wnt-3a (5036-WN), along with the other reagents listed in the intestinal organoid culture medium recipe in the human intestinal organoid culture protocol. (A) Human intestinal organoids were stained using a Rat Anti-Human/Mouse/Rat Vimentin Monoclonal Antibody (MAB2105; green) and a Goat Anti-Human/Mouse Desmin Antigen Affinity-purified Polyclonal Antibody (AF3844; red) to visualize myofibroblast cells and counterstained with DAPI (5748; blue). (B) Human intestinal organoids were stained using a Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (AF748; green) and a Mouse Anti-Human MUC2 Monoclonal Antibody (Novus Biologicals, Catalog # NBP2-44431; red) and counterstained with DAPI (5748; blue).
 View Larger
View Larger
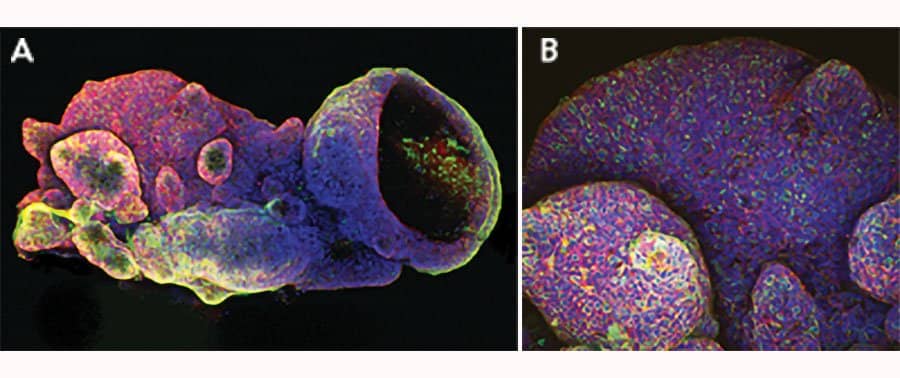
Adult stem cells isolated from human descending colon were embedded in Cultrex UltiMatrix RGF Basement Membrane Extract (BME001-05) and cultured for 30 days in intestinal organoid culture medium, which includes Recombinant Human R-Spondin 1 (Catalog # 4645-RS), Recombinant Human EGF (236-EG), Recombinant Human Noggin (6057-NG), and Recombinant Human Wnt-3a (5036-WN), along with the other reagents listed in the intestinal organoid culture medium recipe in the human intestinal organoid culture protocol. (A) Organoids were fixed and stained with a Mouse Anti-Human MUC2 Monoclonal Antibody (Novus Biologicals; Catalog # NBP2-44431; green) to visualize intestinal goblet cells and counterstained with a Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (AF748; red) and DAPI (5748; blue). The image shown was taken at 10x magnification. (B) Organoids were fixed and stained with a Mouse Anti-Human Chromogranin A Monoclonal Antibody (MAB90981; green) to visualize enteroendocrine cells and counterstained with a Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (AF748; red) and DAPI (5748; blue). The image shown was taken at 20x magnification.
Reconstitution Calculator
Background: R-Spondin 1
R-Spondin 1 (RSPO1, Roof plate-specific Spondin 1), also known as cysteine-rich and single thrombospondin domain containing protein 3 (Cristin 3), is a 27 kDa secreted protein that shares ~40% amino acid (aa) identity with three other R-Spondin family members (1, 2). All R-Spondins regulate Wnt/ beta-Catenin signaling but have distinct expression patterns (1-3). R-Spondin 1 competes with the Wnt antagonist DKK-1 for binding to the Wnt co-receptors, Kremen and LRP-6, reducing their DKK-1-mediated internalization (4). However, reports are mixed on whether R-Spondin 1 binds LRP-6 directly (4-6). R-Spondin 1 is expressed in early development at the roof plate boundary and is thought to contribute to dorsal neural tube development (3, 7). In humans, rare disruptions of the R-Spondin 1 gene are associated with tendencies for XX sex reversal (phenotypic male) or hermaphroditism, indicating a role for R-Spondin 1 in gender-specific differentiation (7, 8). Mutations in R-Spondin 1 are also linked with palmoplantar keratoderma, abnormal thickening of the skin on the palms of the hands and soles of the feet (7, 8). Postnatally, R-Spondin 1 is expressed by neuroendocrine cells in the intestine, adrenal gland and pancreas, and by epithelia in kidney and prostate (9). Injection of recombinant R-Spondin 1 in mice causes activation of beta-catenin and proliferation of intestinal crypt epithelial cells, and ameliorates experimental colitis (9, 10). Interest in R-Spondin 1 as a cell culture supplement has grown with the expansion of the organoid field. R-Spondin 1 is widely used in organoid cell culture workflows as a vital component that promotes both growth and survival of 3D organoids (11).
Structurally similar to other R-Spondins, R-Spondin 1 contains two adjacent cysteine-rich furin-like domains (aa 34-135) with one potential N-glycosylation site, followed by a thrombospondin (TSP-1) motif (aa 147-207) and a region rich in basic residues (aa 211-263). Only the furin-like domains are needed for beta-catenin stabilization (2, 12). A putative nuclear localization signal at the C-terminus may allow some expression in the nucleus (13). Potential isoforms of 200 and 236 aa have an alternate, shorter N-terminus or are missing aa 146-208, respectively (14). Over aa 21-263, human R-Spondin 1 shares 89%, 87%, 92%, 91%, 91% and 89% aa identity with mouse, rat, horse, dog, goat, and cow RSPO-1, respectively.
- Chen, J-Z. et al. (2002) Mol. Biol. Rep. 29:287.
- Kim, K.-A. et al. (2006) Cell Cycle 5:23.
- Nam, J.-S. et al. (2007) Gene Expr. Patterns 7:306.
- Binnerts, M.E. et al. (2007) Proc. Natl. Acad. Sci. USA 104:14700.
- Nam, J.-S. et al. (2006) J. Biol. Chem. 281:13247.
- Wei, Q. et al. (2007) J. Biol. Chem. 282:15903.
- Kamata, T. et al. (2004) Biochim. Biophys. Acta 1676:51.
- Parma, P. et al. (2006) Nat. Genet. 38:1304.
- Kim, K.-A. et al. (2005) Science 309:1256.
- Zhao, J. et al. (2007) Gastroenterology 132:1331.
- Drost and Clevers. (2018) Nature Reviews Cancer 18:407.
- Kazanskaya, O. et al. (2004) Dev. Cell 7:525.
- Tomaselli, S. et al. (2008) Hum. Mutat. 29:220.
- UniProt # Q2MKA7.
Citations for Recombinant Human R-Spondin 1 Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
105
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Mesothelioma-Associated Fibroblasts Modulate the Response of Mesothelioma Patient-Derived Organoids to Chemotherapy via Interleukin-6
Authors: Cioce, M;Gatti, V;Napolitano, F;Giorgiano, NM;Marra, A;Portella, G;Fiorelli, A;Pentimalli, F;Fazio, VM;
International journal of molecular sciences
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neratinib could be effective as monotherapy or in combination with trastuzumab in HER2-low breast cancer cells and organoid models
Authors: Arshad, M;Azad, A;Chan, PYK;Vigneswara, V;Feldinger, K;Nafi, SNM;Laporte-Maguire, E;De Santo, C;Zuo, J;Shaaban, AM;Kong, A;
British journal of cancer
Species: Human
Sample Types: Organoid
Applications: Bioassay -
p53 promotes revival stem cells in the regenerating intestine after severe radiation injury
Authors: Morral, C;Ayyaz, A;Kuo, HC;Fink, M;Verginadis, II;Daniel, AR;Burner, DN;Driver, LM;Satow, S;Hasapis, S;Ghinnagow, R;Luo, L;Ma, Y;Attardi, LD;Koumenis, C;Minn, AJ;Wrana, JL;Lee, CL;Kirsch, DG;
Nature communications
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Tofacitinib uptake by patient-derived intestinal organoids predicts individual clinical responsiveness
Authors: Jang, KK;Ercelen, D;Cen Feng, JYC;Gurunathan, S;Zhou, C;Korman, A;Newell, L;Hudesman, D;Jones, DR;Loke, P;Axelrad, JE;Cadwell, K;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Trophoblast organoids with physiological polarity model placental structure and function
Authors: Yang, L;Liang, P;Yang, H;Coyne, CB;
Journal of cell science
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Establishment of a large-scale patient-derived high-risk colorectal adenoma organoid biobank for high-throughput and high-content drug screening
Authors: Luo, Z;Wang, B;Luo, F;Guo, Y;Jiang, N;Wei, J;Wang, X;Tseng, Y;Chen, J;Zhao, B;Liu, J;
BMC medicine
Species: Human
Sample Types: Organoids
Applications: Bioassay -
GSK3? Regulates Temporally Dynamic Changes in Ribosomal Proteins upon Amino Acid Starvation in Cancer Cells
Authors: Loxha, L;Ibrahim, NK;Stasche, AS;Cinar, B;Dolgner, T;Niessen, J;Schreek, S;Fehlhaber, B;Forster, M;Stanulla, M;Hinze, L;
International journal of molecular sciences
Species: Transgenic Mouse
Sample Types: Organoid
Applications: Bioassay -
Persistent organic pollutants promote aggressiveness in prostate cancer
Authors: Buñay, J;Kossai, M;Damon-Soubeyrant, C;De Haze, A;Saru, JP;Trousson, A;de Joussineau, C;Bouchareb, E;Kocer, A;Vialat, M;Dallel, S;Degoul, F;Bost, F;Clavel, S;Penault-Llorca, F;Valli, MP;Guy, L;Matthews, J;Renaud, Y;Ittmann, M;Jones, J;Morel, L;Lobaccaro, JM;Baron, S;
Oncogene
Species: Mouse
Sample Types: Organoid
Applications: Cell Culture -
Genetic characterization of a novel organoid from human malignant giant-cell tumor
Authors: Suzuki, R;Wakamatsu, T;Yoshida, K;Matsuoka, Y;Takami, H;Nakai, S;Tamiya, H;Kakunaga, S;Yagi, T;Yoshida, KI;Imura, Y;Yui, Y;Sasagawa, S;Takenaka, S;
Journal of bone oncology
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Patterning and folding of intestinal villi by active mesenchymal dewetting
Authors: Huycke, TR;Miyazaki, H;Häkkinen, TJ;Srivastava, V;Barruet, E;McGinnis, CS;Kalantari, A;Cornwall-Scoones, J;Vaka, D;Zhu, Q;Jo, H;DeGrado, WF;Thomson, M;Garikipati, K;Boffelli, D;Klein, OD;Gartner, ZJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoids
Applications: Bioassay -
p53 promotes revival stem cells in the regenerating intestine after severe radiation injury
Authors: Morral, C;Ayyaz, A;Kuo, HC;Fink, M;Verginadis, I;Daniel, AR;Burner, DN;Driver, LM;Satow, S;Hasapis, S;Ghinnagow, R;Luo, L;Ma, Y;Attardi, LD;Koumenis, C;Minn, AJ;Wrana, JL;Lee, CL;Kirsch, DG;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
Simple Detection and Culture of Circulating Tumor Cells from Colorectal Cancer Patients Using Poly(2-Methoxyethyl Acrylate)-Coated Plates
Authors: M Nomura, Y Yokoyama, D Yoshimura, Y Minagawa, A Yamamoto, Y Tanaka, N Sekiguchi, D Marukawa, M Ichihara, H Itakura, K Matsumoto, Y Morimoto, H Tomihara, A Inoue, T Ogino, N Miyoshi, H Takahashi, H Takahashi, M Uemura, S Kobayashi, T Mizushima, T Anada, M Mori, Y Doki, M Tanaka, H Eguchi, H Yamamoto
International Journal of Molecular Sciences, 2023-02-16;24(4):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Combined PD-1, BRAF and MEK inhibition in BRAFV600E colorectal cancer: a phase 2 trial
Authors: J Tian, JH Chen, SX Chao, K Pelka, M Giannakis, J Hess, K Burke, V Jorgji, P Sindurakar, J Braverman, A Mehta, T Oka, M Huang, D Lieb, M Spurrell, JN Allen, TA Abrams, JW Clark, AC Enzinger, PC Enzinger, SJ Klempner, NJ McCleary, JA Meyerhardt, DP Ryan, MB Yurgelun, K Kanter, EE Van Sevent, I Baiev, G Chi, J Jarnagin, WB Bradford, E Wong, AG Michel, IJ Fetter, G Siravegna, AJ Gemma, A Sharpe, S Demehri, R Leary, CD Campbell, O Yilmaz, GA Getz, AR Parikh, N Hacohen, RB Corcoran
Nature Medicine, 2023-01-26;0(0):.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Targeting TBK1 to overcome resistance to cancer immunotherapy
Authors: Y Sun, OY Revach, S Anderson, EA Kessler, CH Wolfe, A Jenney, CE Mills, EJ Robitschek, TGR Davis, S Kim, A Fu, X Ma, J Gwee, P Tiwari, PP Du, P Sindurakar, J Tian, A Mehta, AM Schneider, K Yizhak, M Sade-Feldm, T LaSalle, T Sharova, H Xie, S Liu, WA Michaud, R Saad-Beret, KB Yates, A Iracheta-V, JKE Spetz, X Qin, KA Sarosiek, G Zhang, JW Kim, MY Su, AM Cicerchia, MQ Rasmussen, SJ Klempner, D Juric, SI Pai, DM Miller, A Giobbie-Hu, JH Chen, K Pelka, DT Frederick, S Stinson, E Ivanova, AR Aref, CP Paweletz, DA Barbie, DR Sen, DE Fisher, RB Corcoran, N Hacohen, PK Sorger, KT Flaherty, GM Boland, RT Manguso, RW Jenkins
Nature, 2023-01-12;0(0):.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Multiparametric and accurate functional analysis of genetic sequence variants using CRISPR-Select
Authors: Y Niu, CA Ferreira A, X Li, E Kamali, O Haagen Nie, C Storgaard, M Frödin
Nature Genetics, 2022-12-05;54(12):1983-1993.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Microfluidic Device to Manipulate 3D Human Epithelial Cell-Derived Intestinal Organoids
Authors: M Matsumoto, Y Morimoto, T Sato, S Takeuchi
Micromachines, 2022-11-26;13(12):.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Generation and cryopreservation of feline oviductal organoids
Authors: RE Thompson, MA Meyers, C Premananda, FK Hollinshea
Theriogenology, 2022-11-17;196(0):167-173.
Species: Feline
Sample Types: Organoids
Applications: Bioassay -
Identification of trypsin-degrading commensals in the large intestine
Authors: Y Li, E Watanabe, Y Kawashima, DR Plichta, Z Wang, M Ujike, QY Ang, R Wu, M Furuichi, K Takeshita, K Yoshida, K Nishiyama, SM Kearney, W Suda, M Hattori, S Sasajima, T Matsunaga, X Zhang, K Watanabe, J Fujishiro, JM Norman, B Olle, S Matsuyama, H Namkoong, Y Uwamino, M Ishii, K Fukunaga, N Hasegawa, O Ohara, RJ Xavier, K Atarashi, K Honda
Nature, 2022-09-07;609(7927):582-589.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
Lymphatics act as a signaling hub to regulate intestinal stem cell activity
Authors: RE Niec, T Chu, M Schernthan, S Gur-Cohen, L Hidalgo, HA Pasolli, KA Luckett, Z Wang, SR Bhalla, F Cambuli, RP Kataru, K Ganesh, BJ Mehrara, D Pe'er, E Fuchs
Cell Stem Cell, 2022-06-20;29(7):1067-1082.e18.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids
Authors: S Kim, S Min, YS Choi, SH Jo, JH Jung, K Han, J Kim, S An, YW Ji, YG Kim, SW Cho
Nature Communications, 2022-03-30;13(1):1692.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Elimination of Reprogramming Transgenes Facilitates the Differentiation of Induced Pluripotent Stem Cells into Hepatocyte-like Cells and Hepatic Organoids
Authors: J Jeong, TH Kim, M Kim, YK Jung, KS Kim, S Shim, H Jang, WI Jang, SB Lee, D Choi
Biology, 2022-03-23;11(4):.
Species: Human
Sample Types: Transduced Whole Cells
Applications: Bioassay -
Renalase and its receptor, PMCA4b, are expressed in the placenta throughout the human gestation
Authors: M Wang, T Silva, JM Toothaker, BT McCourt, C Shugrue, G Desir, F Gorelick, L Konnikova
Scientific Reports, 2022-03-23;12(1):4953.
Species: Human
Sample Types: Organoid
Applications: Cell Culture -
Spatiotemporal reprogramming of differentiated cells underlies regeneration and neoplasia in the intestinal epithelium
Authors: T Higa, Y Okita, A Matsumoto, S Nakayama, T Oka, O Sugahara, D Koga, S Takeishi, H Nakatsumi, N Hosen, S Robine, MM Taketo, T Sato, KI Nakayama
Nature Communications, 2022-03-21;13(1):1500.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Establishment of bovine 3D enteroid-derived 2D monolayers
Authors: KM Sutton, B Orr, J Hope, SR Jensen, L Vervelde
Veterinary research, 2022-03-02;53(1):15.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
A beta-Catenin-TCF-Sensitive Locus Control Region Mediates GUCY2C Ligand Loss in Colorectal Cancer
Authors: JA Rappaport, AA Entezari, A Caspi, S Caksa, AV Jhaveri, TJ Stanek, A Ertel, J Kupper, PM Fortina, SB McMahon, JB Jaynes, AE Snook, SA Waldman
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-22;0(0):.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Desmoplakin maintains transcellular keratin scaffolding and protects from intestinal injury
Authors: A Gross, B Zhou, L Bewersdorf, N Schwarz, GM Schacht, P Boor, K Hoeft, B Hoffmann, E Fuchs, R Kramann, R Merkel, RE Leube, P Strnad
Cellular and Molecular Gastroenterology and Hepatology, 2021-12-17;0(0):.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Novel chicken two-dimensional intestinal model comprising all key epithelial cell types and a mesenchymal sub-layer
Authors: B Orr, K Sutton, S Christian, T Nash, H Niemann, LL Hansen, MJ McGrew, SR Jensen, L Vervelde
Veterinary research, 2021-11-24;52(1):142.
Species: Chicken
Sample Types: Whole Cells
Applications: Bioassay -
Lef1 restricts ectopic crypt formation and tumor cell growth in intestinal adenomas
Authors: S Heino, S Fang, M Lähde, J Högström, S Nassiri, A Campbell, D Flanagan, A Raven, M Hodder, N Nasreddin, HH Xue, M Delorenzi, S Leedham, TV Petrova, O Sansom, K Alitalo
Science Advances, 2021-11-17;7(47):eabj0512.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
Intestinal antiviral signaling is controlled by autophagy gene Epg5 independent of the microbiota
Authors: S Lee, G Kalugotla, H Ingle, R Rodgers, C Wu, Y Wang, Y Li, X Yang, J Zhang, NR Borella, H Deng, L Droit, R Hill, ST Peterson, C Desai, D Lawrence, Q Lu, MT Baldridge
Autophagy, 2021-09-14;0(0):1-16.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
A pan-cancer organoid platform for precision medicine
Authors: BM Larsen, M Kannan, LF Langer, BD Leibowitz, A Bentaieb, A Cancino, I Dolgalev, BE Drummond, JR Dry, CS Ho, G Khullar, BA Krantz, B Mapes, KE McKinnon, J Metti, JF Perera, TA Rand, V Sanchez-Fr, JM Shaxted, MM Stein, MA Streit, YC Tan, Y Zhang, E Zhao, J Venkataram, MC Stumpe, JA Borgia, A Masood, DVT Catenacci, JV Mathews, DB Gursel, JJ Wei, TH Welling, DM Simeone, KP White, AA Khan, C Igartua, AA Salahudeen
Cell Reports, 2021-07-27;36(4):109429.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Measuring the elastic modulus of soft culture surfaces and three-dimensional hydrogels using atomic force microscopy
Authors: MDA Norman, SA Ferreira, GM Jowett, L Bozec, E Gentleman
Nature Protocols, 2021-04-14;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Capturing human trophoblast development with naive pluripotent stem cells in�vitro
Authors: S Io, M Kabata, Y Iemura, K Semi, N Morone, A Minagawa, B Wang, I Okamoto, T Nakamura, Y Kojima, C Iwatani, H Tsuchiya, B Kaswandy, E Kondoh, S Kaneko, K Woltjen, M Saitou, T Yamamoto, M Mandai, Y Takashima
Cell Stem Cell, 2021-04-07;28(6):1023-1039.e13.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Comparative Analysis of Patient-Matched PDOs Revealed a Reduction in OLFM4-Associated Clusters in Metastatic Lesions in Colorectal Cancer
Authors: T Okamoto, D duVerle, K Yaginuma, Y Natsume, H Yamanaka, D Kusama, M Fukuda, M Yamamoto, F Perraudeau, U Srivastava, Y Kashima, A Suzuki, Y Kuze, Y Takahashi, M Ueno, Y Sakai, T Noda, K Tsuda, Y Suzuki, S Nagayama, R Yao
Stem Cell Reports, 2021-03-11;0(0):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
LGR5/R-Spo1/Wnt3a axis promotes stemness and aggressive phenotype in hepatoblast-like hepatocellular carcinoma cell lines
Authors: S Akbari, ? Kunter, Y Azbazdar, G Ozhan, N Atabey, ZF Karagonlar, E Erdal
Cellular Signalling, 2021-03-06;0(0):109972.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Murine astrovirus tropism for goblet cells and enterocytes facilitates an IFN-&lambda response in vivo and in enteroid cultures
Authors: H Ingle, E Hassan, J Gawron, B Mihi, Y Li, EA Kennedy, G Kalugotla, H Makimaa, S Lee, P Desai, KG McDonald, MS Diamond, RD Newberry, M Good, MT Baldridge
Mucosal Immunology, 2021-03-05;0(0):.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Farnesyl dimethyl chromanol targets colon cancer stem cells and prevents colorectal cancer metastasis
Authors: K Husain, D Coppola, CS Yang, MP Malafa
Scientific Reports, 2021-01-26;11(1):2185.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Targeted Profiling of Immunological Genes during Norovirus Replication in Human Intestinal Enteroids
Authors: JCM Chan, KN Mohammad, LY Zhang, SH Wong, MC Chan
Viruses, 2021-01-21;13(2):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity
Authors: SK Sarvestani, S Signs, B Hu, Y Yeu, H Feng, Y Ni, DR Hill, RC Fisher, S Ferrandon, RK DeHaan, J Stiene, M Cruise, TH Hwang, X Shen, JR Spence, EH Huang
Nature Communications, 2021-01-11;12(1):262.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Using systems medicine to identify a therapeutic agent with potential for repurposing in inflammatory bowel disease
Authors: K Lloyd, S Papoutsopo, E Smith, P Stegmaier, F Bergey, L Morris, M Kittner, H England, D Spiller, MHR White, CA Duckworth, BJ Campbell, V Poroikov, VAP Martins Do, A Kel, W Muller, DM Pritchard, C Probert, MD Burkitt, SysmedIBD
Dis Model Mech, 2020-11-27;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
The Fragile X Mental Retardation Protein regulates RIP1K and colorectal cancer resistance to necroptosis
Authors: A Di Grazia, I Marafini, G Pedini, D Di Fusco, F Laudisi, V Dinallo, E Rosina, C Stolfi, E Franzè, P Sileri, G Sica, G Monteleone, C Bagni, I Monteleone
Cell Mol Gastroenterol Hepatol, 2020-10-19;0(0):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Aspirin Rescues Wnt-Driven Stem-like Phenotype in Human Intestinal Organoids and Increases the Wnt Antagonist Dickkopf-1
Authors: K Dunbar, A Valanciute, A Lima, PF Vinuela, T Jamieson, V Rajasekara, J Blackmur, AM Ochocka-Fo, A Guazzelli, P Cammareri, MJ Arends, OJ Sansom, KB Myant, SM Farrington, MG Dunlop, FVN Din
Cell Mol Gastroenterol Hepatol, 2020-09-22;0(0):.
Species: Human
Sample Types: Organoid
Applications: Cell Culture -
Telomere dysfunction activates YAP1 to drive tissue inflammation
Authors: D Chakravart, B Hu, X Mao, A Rashid, J Li, J Li, WT Liao, EM Whitley, P Dey, P Hou, KA LaBella, A Chang, G Wang, DJ Spring, P Deng, D Zhao, X Liang, Z Lan, Y Lin, S Sarkar, C Terranova, YL Deribe, SE Blutt, P Okhuysen, J Zhang, E Vilar, OH Nielsen, A Dupont, M Younes, KR Patel, NF Shroyer, K Rai, MK Estes, YA Wang, AA Bertuch, RA DePinho
Nat Commun, 2020-09-21;11(1):4766.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
LSD1 represses a neonatal/reparative gene program in adult intestinal epithelium
Authors: RT Zwiggelaar, HT Lindholm, M Fosslie, M Terndrup P, Y Ohta, A Díez-Sánch, M Martín-Alo, J Ostrop, M Matano, N Parmar, E Kvaløy, RR Spanjers, K Nazmi, M Rye, F Drabløs, C Arrowsmith, J Arne Dahl, KB Jensen, T Sato, MJ Oudhoff
Science Advances, 2020-09-11;6(37):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Establishment and differentiation of long-term trophoblast organoid cultures from the human placenta
Authors: MA Sheridan, RC Fernando, L Gardner, MS Hollinshea, GJ Burton, A Moffett, MY Turco
Nat Protoc, 2020-09-09;0(0):.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Implementing cell-free DNA of pancreatic cancer patient-derived organoids for personalized oncology
Authors: Z Dantes, HY Yen, N Pfarr, C Winter, K Steiger, A Muckenhube, A Hennig, S Lange, T Engleitner, R Öllinger, R Maresch, F Orben, I Heid, G Kaissis, K Shi, G Topping, F Stögbauer, M Wirth, K Peschke, A Papargyrio, M Rezaee-Ogh, K Feldmann, AP Schäfer, R Ranjan, C Lubeseder-, DE Stange, T Welsch, M Martignoni, GO Ceyhan, H Friess, A Herner, L Liotta, M Treiber, G von Figura, M Abdelhafez, P Klare, C Schlag, H Algül, J Siveke, R Braren, G Weirich, W Weichert, D Saur, R Rad, RM Schmid, G Schneider, M Reichert
JCI Insight, 2020-08-06;5(15):.
Species: Human
Sample Types: Cell Lysates, Organoid
Applications: Bioassay, Cell Culture -
Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential
Authors: SI Mae, M Ryosaka, S Sakamoto, K Matsuse, A Nozaki, M Igami, R Kabai, A Watanabe, K Osafune
Cell Rep, 2020-07-28;32(4):107963.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing
Authors: Y Nanki, T Chiyoda, A Hirasawa, A Ookubo, M Itoh, M Ueno, T Akahane, K Kameyama, W Yamagami, F Kataoka, D Aoki
Sci Rep, 2020-07-28;10(1):12581.
Species: Human
Sample Types: Organoid
Applications: Bioassay -
Understanding the regulatory mechanisms of endometrial cells on activities of endometrial mesenchymal stem-like cells during menstruation
Authors: S Xu, RWS Chan, T Li, EHY Ng, WSB Yeung
Stem Cell Res Ther, 2020-06-17;11(1):239.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Generation of intestinal organoids derived from human pluripotent stem cells for drug testing
Authors: S Yoshida, H Miwa, T Kawachi, S Kume, K Takahashi
Sci Rep, 2020-04-06;10(1):5989.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Targeting the Wnt signaling pathway through R-spondin 3 identifies an anti-fibrosis treatment strategy for multiple organs
Authors: M Zhang, M Haughey, NY Wang, K Blease, AM Kapoun, S Couto, I Belka, T Hoey, M Groza, J Hartke, B Bennett, J Cain, A Gurney, B Benish, P Castiglion, C Drew, J Lachowicz, L Carayannop, SD Nathan, J Distler, DA Brenner, K Hariharan, H Cho, W Xie
PLoS ONE, 2020-03-11;15(3):e0229445.
Species: Human
Sample Types: Whole Cells
Applications: IHC Control -
Standardized GMP-compliant scalable production of human pancreas organoids
Authors: M Dossena, R Piras, A Cherubini, M Barilani, E Dugnani, F Salanitro, T Moreth, F Pampaloni, L Piemonti, L Lazzari
Stem Cell Res Ther, 2020-03-04;11(1):94.
Species: Human
Sample Types: Whole Tissue
Applications: Tissue Culture -
Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells
Authors: NJ Hogrebe, P Augsornwor, KG Maxwell, L Velazco-Cr, JR Millman
Nat. Biotechnol., 2020-02-24;0(0):.
Species: Human
Sample Types: cell culture
Applications: Cell Culture -
HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells
Authors: Y Wang, L Lifshitz, K Gellatly, CL Vinton, K Busman-Sah, S McCauley, P Vangala, K Kim, A Derr, S Jaiswal, A Kucukural, P McDonel, PW Hunt, T Greenough, J Houghton, M Somsouk, JD Estes, JM Brenchley, M Garber, SG Deeks, J Luban
Nat. Immunol., 2020-02-17;21(3):274-286.
Species: Human
Sample Types: Cells
Applications: Cell Culture -
Production, purification and characterization of recombinant human R-spondin1 (RSPO1) protein stably expressed in human HEK293 cells
Authors: G Levin, BAA Koga, GG Belchior, ACO Carreira, MC Sogayar
BMC Biotechnol., 2020-01-20;20(1):5.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Comparison of Surgical and Cadaveric Intestine as a Source of Crypt Culture in Humans
Authors: A Scott, B Olack, JD Rouch, HA Khalil, BA Kokubun, NY Lei, J Wang, S Solorzano, M Lewis, JCY Dunn, MG Stelzner, JC Niland, MG Martín
Cell Transplant, 2020-01-01;29(0):9636897209037.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
Endometrial Axin2+ Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation
Authors: SM Syed, M Kumar, A Ghosh, F Tomasetig, A Ali, RM Whan, D Alterman, PS Tanwar
Cell Stem Cell, 2019-12-26;26(1):64-80.e13.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
A cancer rainbow mouse for visualizing the functional genomics of oncogenic clonal expansion
Authors: PG Boone, LK Rochelle, JD Ginzel, V Lubkov, WL Roberts, PJ Nicholls, C Bock, ML Flowers, RJ von Furste, BR Stripp, P Agarwal, AD Borowsky, RD Cardiff, LS Barak, MG Caron, HK Lyerly, JC Snyder
Nat Commun, 2019-12-02;10(1):5490.
Species: Mouse
Sample Types: Organoids
Applications: Cell Culture -
Robust, Long-Term Culture of Endoderm-Derived Hepatic Organoids for Disease Modeling
Authors: S Akbari, GG Sevinç, N Ersoy, O Basak, K Kaplan, K Sevinç, E Ozel, B Sengun, E Enustun, B Ozcimen, A Bagriyanik, N Arslan, TT Önder, E Erdal
Stem Cell Reports, 2019-09-12;13(4):627-641.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
NOD2 Supports Crypt Survival and Epithelial Regeneration after Radiation-Induced Injury
Authors: C Lee, C Choi, HS Kang, SW Shin, SY Kim, HC Park, SN Hong
Int J Mol Sci, 2019-09-02;20(17):.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
USP9X Deubiquitylates DVL2 to Regulate WNT Pathway Specification
Authors: CP Nielsen, KK Jernigan, NL Diggins, DJ Webb, JA MacGurn
Cell Rep, 2019-07-23;28(4):1074-1089.e5.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Mist1 Expression is Required for Paneth Cell Maturation
Authors: CM Dekaney, S King, B Sheahan, J Cortes
Cell Mol Gastroenterol Hepatol, 2019-07-19;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders
Authors: K Shik Mun, K Arora, Y Huang, F Yang, S Yarlagadda, Y Ramananda, M Abu-El-Hai, JJ Palermo, BN Appakalai, JD Nathan, AP Naren
Nat Commun, 2019-07-16;10(1):3124.
Species: Human
Sample Types: Whole Cells
Applications: Cell Culture -
Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue
Authors: OC Tysoe, AW Justin, T Brevini, SE Chen, KT Mahbubani, AK Frank, H Zedira, E Melum, K Saeb-Parsy, AE Markaki, L Vallier, F Sampazioti
Nat Protoc, 2019-05-20;14(6):1884-1925.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Organoid culture media formulated with growth factors of defined cellular activity
Authors: M Urbischek, H Rannikmae, T Foets, K Ravn, M Hyvönen, M de la Roch
Sci Rep, 2019-04-17;9(1):6193.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling
Authors: HLX Wong, HY Qin, SW Tsang, X Zuo, S Che, CFW Chow, X Li, HT Xiao, L Zhao, T Huang, CY Lin, HY Kwan, T Yang, FM Longo, A Lyu, ZX Bian
Nat Commun, 2019-04-15;10(1):1745.
Species: Human, Mouse
Sample Types: Organoids
Applications: Bioassay -
Chemically defined conditions for long-term maintenance of pancreatic progenitors derived from human induced pluripotent stem cells
Authors: S Konagaya, H Iwata
Sci Rep, 2019-01-24;9(1):640.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Pancreatic Cell Fate Determination Relies on Notch Ligand Trafficking by NFIA
Authors: MA Scavuzzo, J Chmielowie, D Yang, K Wamble, LS Chaboub, L Duraine, B Tepe, SM Glasgow, BR Arenkiel, C Brou, B Deneen, M Borowiak
Cell Rep, 2018-12-26;25(13):3811-3827.e7.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Single-Cell Analysis Identifies LY6D as a Marker Linking Castration-Resistant Prostate Luminal Cells to Prostate Progenitors and Cancer
Authors: JD Barros-Sil, DE Linn, I Steiner, G Guo, A Ali, H Pakula, G Ashton, I Peset, M Brown, NW Clarke, RT Bronson, GC Yuan, SH Orkin, Z Li, E Baena
Cell Rep, 2018-12-18;25(12):3504-3518.e6.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery
Authors: R Cruz-Acuña, M Quirós, S Huang, D Siuda, JR Spence, A Nusrat, AJ García
Nat Protoc, 2018-09-01;0(0):.
Species: Human
Sample Types: Organoids
Applications: Bioassay -
IL22 Inhibits Epithelial Stem Cell Expansion in an Ileal Organoid Model
Authors: B Zwarycz, AD Gracz, KR Rivera, IA Williamson, LA Samsa, J Starmer, MA Daniele, L Salter-Cid, Q Zhao, ST Magness
Cell Mol Gastroenterol Hepatol, 2018-07-04;7(1):1-17.
Species: Mouse
Sample Types: Organoids
Applications: Bioassay -
Differential activities and mechanisms of the four R-Spondins in potentiating Wnt/?-catenin signaling
Authors: S Park, J Cui, WA Yu, L Wu, K Carmon, QJ Liu
J. Biol. Chem., 2018-05-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Culture and characterization of chicken small intestinal crypts
Authors: J Li, J Li, SY Zhang, RX Li, X Lin, YL Mi, CQ Zhang
Poult. Sci., 2018-05-01;0(0):.
Species: Avian - Chicken
Sample Types: Whole Cells
Applications: Bioassay -
ATOH1/RFX1/RFX3 transcription factors facilitate the differentiation and characterisation of inner ear hair cell-like cells from patient-specific induced pluripotent stem cells harbouring A8344G mutation of mitochondrial DNA
Authors: YC Chen, CL Tsai, YH Wei, YT Wu, WT Hsu, HC Lin, YC Hsu
Cell Death Dis, 2018-04-01;9(4):437.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
R-spondins can potentiate WNT signaling without LGRs
Authors: AM Lebensohn, R Rohatgi
Elife, 2018-02-06;7(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Cell-type specific potent Wnt signaling blockade by bispecific antibody
Authors: NK Lee, Y Zhang, Y Su, S Bidlingmai, DW Sherbenou, KD Ha, B Liu
Sci Rep, 2018-01-15;8(1):766.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression
Authors: A Fumagalli, SJE Suijkerbui, H Begthel, E Beerling, KC Oost, HJ Snippert, J van Rheene, J Drost
Nat Protoc, 2018-01-04;13(2):235-247.
Species: Human, Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Higher-Order Kidney Organogenesis from Pluripotent Stem Cells
Authors: A Taguchi, R Nishinakam
Cell Stem Cell, 2017-11-09;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
R-Spondin1/LGR5 Activates TGF? Signaling and Suppresses Colon Cancer Metastasis
Authors: X Zhou, L Geng, D Wang, H Yi, G Talmon, J Wang
Cancer Res., 2017-09-22;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Establishment of a refined culture method for rat colon organoids
Authors: H Isshiki, Y Arimura, K Nagaishi, K Kawakami, K Onodera, K Yamashita, Y Naishiro, M Fujimiya, K Imai, Y Shinomura
Biochem. Biophys. Res. Commun., 2017-05-27;0(0):.
Species: Rat
Sample Types: Whole Cells
Applications: Bioassay -
Ring finger protein 43 associates with gastric cancer progression and attenuates the stemness of gastric cancer stem-like cells via the Wnt-?/catenin signaling pathway
Authors: Y Gao, A Cai, H Xi, J Li, W Xu, Y Zhang, K Zhang, J Cui, X Wu, B Wei, L Chen
Stem Cell Res Ther, 2017-04-26;8(1):98.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Directional delivery of RSPO1 by mesenchymal stem cells ameliorates radiation-induced intestinal injury
Authors: W Chen, S Ju, T Lu, Y Xu, X Zheng, H Wang, Y Ge, S Ju
Cytokine, 2017-02-20;95(0):27-34.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Long-term culture-induced phenotypic difference and efficient cryopreservation of small intestinal organoids by treatment timing of Rho kinase inhibitor
Authors: SH Han, S Shim, MJ Kim, HY Shin, WS Jang, SJ Lee, YW Jin, SS Lee, SB Lee, S Park
World J. Gastroenterol, 2017-02-14;23(6):964-975.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Id2 controls specification of Lgr5+ intestinal stem cell progenitors during gut development
Authors: L Nigmatulli, M Norkin, MM Dzama, B Messner, S Sayols, N Soshnikova
EMBO J., 2017-01-11;36(7):869-885.
Species: Mouse
Sample Types: Organoid
Applications: Bioassay -
Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling.
Authors: Lebensohn A, Dubey R, Neitzel L, Tacchelly-Benites O, Yang E, Marceau C, Davis E, Patel B, Bahrami-Nejad Z, Travaglini K, Ahmed Y, Lee E, Carette J, Rohatgi R
Elife, 2016-12-20;5(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
PGE2 is a direct and robust mediator of anion/fluid secretion by human intestinal epithelial cells
Sci Rep, 2016-11-09;6(0):36795.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Intestinal enteroids model GUCY2C-dependent secretion induced by heat-stable enterotoxins
Infect Immun, 2016-09-19;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
P2X7 receptor-dependent tuning of gut epithelial responses to infection
Authors: SW Huang, C Walker, J Pennock, K Else, W Muller, MJ Daniels, C Pellegrini, D Brough, G Lopez-Cast, SM Cruickshan
Immunol. Cell Biol, 2016-08-25;95(2):178-188.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
Novel Bi-specific Domain Antibody to LRP6 Inhibits Wnt and R-spondin Ligand-induced Wnt Signaling and Tumor Growth
Mol Cancer Res, 2016-07-11;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Development of Functional Microfold (M) Cells from Intestinal Stem Cells in Primary Human Enteroids.
Authors: Rouch J, Scott A, Lei N, Solorzano-Vargas R, Wang J, Hanson E, Kobayashi M, Lewis M, Stelzner M, Dunn J, Eckmann L, Martin M
PLoS ONE, 2016-01-28;11(1):e0148216.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Therapeutic Targeting of Tumor-Derived R-Spondin Attenuates beta-Catenin Signaling and Tumorigenesis in Multiple Cancer Types.
Authors: Chartier C, Raval J, Axelrod F, Bond C, Cain J, Dee-Hoskins C, Ma S, Fischer M, Shah J, Wei J, Ji M, Lam A, Stroud M, Yen W, Yeung P, Cancilla B, O'Young G, Wang M, Kapoun A, Lewicki J, Hoey T, Gurney A
Cancer Res, 2015-12-30;76(3):713-23.
Species: Mouse
Sample Types: In Vivo
Applications: Bioassay -
Characterization and propagation of tumor initiating cells derived from colorectal liver metastases: trials, tribulations and a cautionary note.
Authors: James M, Howells L, Karmokar A, Higgins J, Greaves P, Cai H, Dennison A, Metcalfe M, Garcea G, Lloyd D, Berry D, Steward W, Brown K
PLoS ONE, 2015-02-06;10(2):e0117776.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium.
Authors: Jabaji Z, Brinkley G, Khalil H, Sears C, Lei N, Lewis M, Stelzner M, Martin M, Dunn J
PLoS ONE, 2014-09-15;9(9):e107814.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Luminal microbes promote monocyte-stem cell interactions across a healthy colonic epithelium.
Authors: Skoczek D, Walczysko P, Horn N, Parris A, Clare S, Williams M, Sobolewski A
J Immunol, 2014-06-06;193(1):439-51.
Species: Human
Sample Types: Whole Tissue
Applications: Bioassay -
Hippo pathway activity influences liver cell fate.
Authors: Yimlamai D, Christodoulou C, Galli G, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger B, Camargo F
Cell, 2014-06-05;157(6):1324-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
RSPO-LGR4 functions via IQGAP1 to potentiate Wnt signaling.
Authors: Carmon K, Gong X, Yi J, Thomas A, Liu Q
Proc Natl Acad Sci U S A, 2014-03-17;111(13):E1221-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Inner ear hair cell-like cells from human embryonic stem cells.
Authors: Ronaghi, Mohammad, Nasr, Marjan, Ealy, Megan, Durruthy-Durruthy, Robert, Waldhaus, Joerg, Diaz, Giovanni, Joubert, Lydia-Ma, Oshima, Kazuo, Heller, Stefan
Stem Cells Dev, 2014-03-10;23(11):1275-84.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Modulation of stemness in a human normal intestinal epithelial crypt cell line by activation of the WNT signaling pathway.
Authors: Guezguez A, Pare F, Benoit Y, Basora N, Beaulieu J
Exp Cell Res, 2014-02-15;322(2):355-64.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells.
Authors: Lei N, Jabaji Z, Wang J, Joshi V, Brinkley G, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, Dunn J, Martin M
PLoS ONE, 2014-01-06;9(1):e84651.
Species: Mouse
Sample Types: Whole Tissue
Applications: Bioassay -
RSPO2-LGR5 signaling has tumour-suppressive activity in colorectal cancer.
Authors: Wu C, Qiu S, Lu L, Zou J, Li W, Wang O, Zhao H, Wang H, Tang J, Chen L, Xu T, Sun Z, Liao W, Luo G, Lu X
Nat Commun, 2014-01-01;5(0):3149.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells.
Authors: Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann J, Miyazaki S, Miyazaki J, Szot G, Bottino R, Kim S
Elife, 2013-11-19;2(0):e00940.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Neuregulin autocrine signaling promotes self-renewal of breast tumor-initiating cells by triggering HER2/HER3 activation.
Authors: Lee, Cleo Yi-, Lin, Yuan, Bratman, Scott V, Feng, Weiguo, Kuo, Angera H, Scheeren, Ferenc A, Engreitz, Jesse M, Varma, Sushama, West, Robert B, Diehn, Maximili
Cancer Res, 2013-10-31;74(1):341-52.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Authors: Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J
Nature, 2012-07-26;487(7408):505-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Role of Gadd45a in Wip1-dependent regulation of intestinal tumorigenesis.
Cell Death Differ., 2012-05-04;19(11):1761-8.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner.
Authors: Hao HX, Xie Y, Zhang Y, Charlat O, Oster E, Avello M, Lei H, Mickanin C, Liu D, Ruffner H, Mao X, Ma Q, Zamponi R, Bouwmeester T, Finan PM, Kirschner MW, Porter JA, Serluca FC, Cong F
Nature, 2012-04-29;485(7397):195-200.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay -
Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro.
Authors: Spence JR, Mayhew CN, Rankin SA
Nature, 2010-12-12;470(7332):105-9.
Species: Human
Sample Types: Whole Cells
Applications: Bioassay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human R-Spondin 1 Protein
Average Rating: 4.6 (Based on 7 Reviews)
Have you used Recombinant Human R-Spondin 1 Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: R-spondin was used as indicated by the manufacturer and stored in aliquots. The product was easy to dissolve and allowed us replicable and excellent results in 3D culture
R-Spondin was used for colonocyte isolation and culturing from Sprague-Dawley rats in 3D culture. Results showed a purified culture as spheroids-like, following a standard isolation protocol for these cells
Used for the differentiation of iPSC into intestinal organoids, at a concentration of 500ng/mL
Reason for Rating: stable product when used as described; produces repeatable results for differentiation
Reason for Rating: Protein performed as expected in assay
Wnt3a biology and cell growth. Use HEK Wnt3a reporter cell line.