Recombinant Human Leptin R Fc Chimera Protein Summary
Product Specifications
| Human Leptin R (Thr20-Asp839) Accession # P48357.2 |
IIEGRDMD | Human IgG1 (Pro100-Lys330) |
6-His tag |
| N-terminus | C-terminus | ||
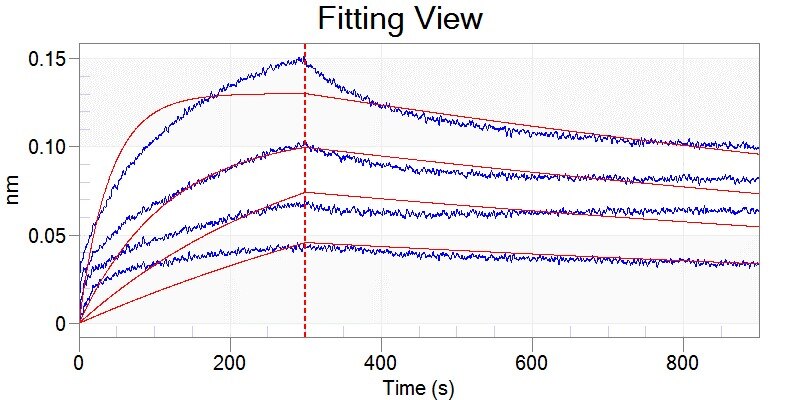
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
389-LR
| Formulation | Lyophilized from a 0.2 μm filtered solution in MES, NaCl and CHAPS with BSA as a carrier protein. |
| Reconstitution | Reconstitute at 200 μg/mL in sterile PBS containing at least 0.1% human or bovine serum albumin. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
389-LR/CF
| Formulation | Lyophilized from a 0.2 μm filtered solution in MES, NaCl and CHAPS. |
| Reconstitution | Reconstitute at 200 μg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Reconstitution Calculator
Background: Leptin R
The Leptin receptor (Leptin R, gene name LEPR), also called OB R (obesity receptor), is a 150 kDa protein that is a member of the Class I cytokine receptor family. It mediates the activities of Leptin, a multi-functional hormone produced primarily by adipose tissues that plays roles in food intake, energy metabolism, angiogenesis, reproduction, hematopoiesis, bone metabolism, and immune function (1-3). The human Leptin R gene encodes 1165 amino acids (aa) including a signal peptide, an extracellular region with cytokine receptor homology (CRH), multiple fibronectin type III domains and a WSXWS motif, a transmembrane domain, and a cytoplasmic domain that supports JAK/STAT signaling (2, 3). Human Leptin R shares 76% aa sequence identity with mouse and rat Leptin R, and 83-86% with bovine, canine, equine and porcine Leptin R. Leptin R isoforms include a long form, OB RL or OB Rb (primary signaling form), and at least four shorter isoforms with truncated cytoplasmic domains, named OB Ra (ubiquitous), Rc, Rd, and Rf (2, 4). A soluble isoform, OB Re, is found in rodents but not humans (5). However, both humans and rodents produce soluble Leptin R due to release of soluble ectodomains by metalloproteinases such as ADAM10 (5, 6). OB Rb is highly expressed in the hypothalamus and mediates the anti-orexigenic effects of Leptin (1, 2). Mutations of ObRb have caused extreme obesity in humans, mice (db/db “diabetes”), and rats (Zucker fa/fa “fatty”) (1, 7-9). Shorter isoforms of Leptin R exhibit limited signaling capability, but mediate endocytosis and degradation of Leptin and passage through the blood-brain barrier (4, 5, 10, 11). Soluble Leptin R is the primary Leptin-binding protein in blood, where it maintains a pool of available bioactive Leptin, delays Leptin clearance from circulation, and down-regulates blood-brain transmission of Leptin (5-7, 10). In humans, soluble Leptin R levels are inversely proportional to adiposity and are elevated in females versus males (12). Soluble Leptin R is also found up-regulated in patients with chronic heart failure, end-stage renal disease, and anorexia (13-15). It is expressed by tumor-initiating stem cells, and is proposed as a link between between cancer and obesity (16).
- Israel, D. and S. Chua, Jr. (2010) Trends Endocrinol. Metab. 21:10.
- Oswal, A. and G. Yeo (2010) Obesity 18:221.
- Tartaglia, L.A. et al. (1995) Cell 83:1263.
- Murakami, T. et al. (1997) Biochem. Biophys. Res. Commun. 231:26.
- Lou, P.H. et al. (2010) PLoS ONE 5:e11669.
- Schaab, M. et al. (2012) PLoS ONE 7:e34787.
- Huang, L. et al. (2001) J. Biol. Chem. 276:6343.
- Chen, H. et al. (1996) Cell 84:491.
- Phillips, M.S. et al. (1996) Nature Genet. 13:18.
- Tu, H. et al. (2008) J. Cell Physiol. 214:301.
- Tu, H. et al. (2007) J. Cell. Physiol. 212:215.
- Mann, D.R. et al. (2003) J. Clin. Endocrinol. Metab. 88:3339.
- Schulze, P.C. et al. (2003) Eur. J. Heart Fail. 5:33.
- Pecoits-Filho, R. et al. (2002) Eur. J. Clin. Invest. 32:811.
- Krizova, J. et al. (2002) Endocr. Res. 28:199.
- Feldman, D.E. et al. (2012) Proc. Natl. Acad. Sci. USA 109:829.
Citations for Recombinant Human Leptin R Fc Chimera Protein
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
4
Citations: Showing 1 - 4
Filter your results:
Filter by:
-
Enhancing Oral Delivery of Biologics: A Non-Competitive and Cross-Reactive Anti-Leptin Receptor Nanofitin Demonstrates a Gut-Crossing Capacity in an Ex Vivo Porcine Intestinal Model
Authors: Masloh, S;Chevrel, A;Culot, M;Perrocheau, A;Kalia, YN;Frehel, S;Gaussin, R;Gosselet, F;Huet, S;Zeisser Labouebe, M;Scapozza, L;
Pharmaceutics
Species: Human
Sample Types: Recombinant Protein
Applications: Bioassay -
Intranasal delivery of N-terminal modified leptin-pluronic conjugate for treatment of obesity
Authors: D Yuan, X Yi, Y Zhao, CD Poon, KM Bullock, KM Hansen, TS Salameh, SA Farr, WA Banks, AV Kabanov
J Control Release, 2017-03-24;0(0):.
Applications: Bioassay -
BiaCore analysis of leptin-leptin receptor interaction: evidence for 1:1 stoichiometry.
Authors: Mistrik P, Moreau F, Allen JM
Anal. Biochem., 2004-04-15;327(2):271-7.
Species: Human, Mouse, Rat
Sample Types: Recombinant Protein
Applications: Surface Plasmon Resonance -
Steps toward mapping the human vasculature by phage display.
Authors: Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R
Nat. Med., 2002-02-01;8(2):121-7.
Species: Human
Sample Types: Buffer
Applications: Binding Assay
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human Leptin R Fc Chimera Protein
Average Rating: 4 (Based on 1 Review)
Have you used Recombinant Human Leptin R Fc Chimera Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: