Mouse VE-Cadherin Antibody Summary
Asp46-Gln592
Accession # 2208309A
*Small pack size (-SP) is supplied either lyophilized or as a 0.2 µm filtered solution in PBS.
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Mouse VE‑Cadherin by Western Blot. Western blot shows lysates of mouse lung tissue and bEnd.3 mouse endothelioma cell line. PVDF membrane was probed with 0.2 µg/mL of Goat Anti-Mouse VE‑Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1002) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for VE‑Cadherin at approximately 120 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
VE‑Cadherin in Mouse Heart. VE-Cadherin was detected in immersion fixed paraffin-embedded sections of mouse heart using Goat Anti-Mouse VE-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1002) at 3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell membranes. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Mouse VE‑Cadherin by Simple WesternTM. Simple Western lane view shows lysates of mouse lung tissue, loaded at 0.2 mg/mL. Specific bands were detected for VE‑Cadherin at approximately 118, 146 kDa (as indicated) using 10 µg/mL of Goat Anti-Mouse VE‑Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF1002). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Rat VE-Cadherin by Immunohistochemistry Chronic intermittent hypoxia modifies the BM vascular structure. a’–e’ Representative images of femur bone marrow stained with vWF, CD105, VE-cadherin, SMA, and CD11b counterstained with hematoxylin. a”, c”, d” BM from CIH exposed rats (n = 6) has more VE-cadherin+ vessels and SMA coverage but less vWF+ sinusoids (400×, Leica DM2500). e’, e” Representative images of CD11b immunohistochemistry in femur BM show an increase in BM monocyte count in CIH exposed animals. (400×, Leica DM2500) a’, a”’, b’, b” No changes in the total number of vessels or in megakaryocyte count were observed, as accounted by CD105 and vWF staining, respectively. Results are represented as the mean ± SD of bone marrow sections from six male Wistar rats (*p < 0.05; **p < 0.01). f Representative images of femur bone marrow fluorescently immunostained for VE-cadherin show an increase in total VE-cadherin vessels and in VE-cadherin vessel coverage. Scale bar, 50 μm (insets magnified 2.5×). Images were acquired with a Zeiss LSM 510 META microscope Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26856724), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Flow Cytometry FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Flow Cytometry FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Flow Cytometry FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Rat VE-Cadherin by Immunohistochemistry Chronic intermittent hypoxia modifies the BM vascular structure. a’–e’ Representative images of femur bone marrow stained with vWF, CD105, VE-cadherin, SMA, and CD11b counterstained with hematoxylin. a”, c”, d” BM from CIH exposed rats (n = 6) has more VE-cadherin+ vessels and SMA coverage but less vWF+ sinusoids (400×, Leica DM2500). e’, e” Representative images of CD11b immunohistochemistry in femur BM show an increase in BM monocyte count in CIH exposed animals. (400×, Leica DM2500) a’, a”’, b’, b” No changes in the total number of vessels or in megakaryocyte count were observed, as accounted by CD105 and vWF staining, respectively. Results are represented as the mean ± SD of bone marrow sections from six male Wistar rats (*p < 0.05; **p < 0.01). f Representative images of femur bone marrow fluorescently immunostained for VE-cadherin show an increase in total VE-cadherin vessels and in VE-cadherin vessel coverage. Scale bar, 50 μm (insets magnified 2.5×). Images were acquired with a Zeiss LSM 510 META microscope Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/26856724), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Flow Cytometry FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence Resting skin vessels contain high density HS moieties preferentially expressed at their basolateral compartments.(A, C, E, F, G) Axial and longitudinal sections of vessels of (A, B) naïve frozen, or (C, D, E, F, G) paraffin embedded murine flank skin tissues. Tissues were stained for HS with monoclonal mouse IgM (10E4 epitope; red), goat anti mouse VE-cadherin (cyan), FITC-mouse monoclonal to alpha -SMA (green), and nuclei (blue). (H) HS and alpha -SMA profile of the fluorescence intensity along the purple arrow in the merged image. Double-headed black arrow denotes the vessel lumen as indicated by VE-cadherin. In C, D and G, small-headed arrows denote the endothelial apical side, large-headed arrows denote the endothelial basolateral side, and broken-line arrows denote the pericyte basolateral side. Images were taken at 100X magnification. Scale bar represents 5 µm. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085699), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunocytochemistry/Immunofluorescence FACS analysis of emerging vascular EC progenies from mES and iPS cells.Adherent cells (2x105) were detached and subjected to two-step FACS-aided purification. Control FACS profile on day 5 of cells derived from mES (J1) cells (A). Representative FACS profiles of day 5, with vascular progenies assessed using anti-Flk1 and anti-VE-cadherin antibodies obtained from mES (J1) cells (B) and derived from miPS (iMZ-21) cells (C); Control FACS profile on day 5 of cells derived from miPS (iMZ-21) cells (D). Representative FACS after the second step of purification derived from mES (E) and iPS cells (F). The yield of Flk1+VE-cadherin+ after the second round of FACS was 100% for both mES and miPS-derived vascular progenies. Morphology of mES- and miPS-derived vascular ECs (G-J). Flk1+VE-cadherin+ vascular progenies derived from mES and miPS cells were cultured overnight in IV Col-coated dishes, immunostained with anti-VE-cadherin (green) and anti-CD31 (red) of cells derived from mES cells (G&H) and miPS cells (I&J). DAPI, nucleus (blue). Magnifications are as indicated; the scale bar is 200 µm. Experiments were repeated 3 times. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0085549), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of VE‑Cadherin in Mouse Heart. Formalin-fixed paraffin-embedded tissue sections of mouse heart were probed for VE-Cadherin mRNA (ACD RNAScope Probe, catalog #312538; Fast Red chromogen, ACD catalog # 322750). Adjacent tissue section was processed for immunohistochemistry using goat anti-mouse VE-Cadherin polyclonal antibody (R&D Systems catalog # AF1002) at 3ug/mL with 1 hour incubation at room temperature followed by incubation with anti-goat IgG VisUCyte HRP Polymer Antibody (Catalog # VC004) and DAB chromogen (yellow-brown). Tissue was counterstained with hematoxylin (blue). Specific staining was localized to cardiac myocytes.
 View Larger
View Larger
Detection of Mouse Mouse VE-Cadherin Antibody by Western Blot Warfarin pretreatment enhances tumor Ad5ROBO4 vector expression.A. Multiorgan immunoblot of vehicle (left lanes) or warfarin (right lanes) pretreated Rag2−/− mice injected with 1.0×1011 vp of Ad5ROBO4. B. Densitometry of A revealed that vehicle pretreatment was associated with robust liver, detectable splenic, and trace to undetectable expression in all other sampled organs. Warfarin pretreatment produced a 2.5-fold increased splenic and a 3-fold decreased liver expression while all other organs still evidenced trace to undetectable expression. C. Immunoblot and densitometry of liver and tumor EGFP, VE-cadherin, and beta -tubulin expression in vehicle (left lanes) or warfarin (right lanes) from the same pretreated, Ad5ROBO4-injected mice as in A and B. EGFP densitometry normalized to VE-cadherin, revealed a 4.7-fold decrease in liver and 2-fold increase in increase KO and SC tumor expression produced by warfarin pretreatment. A–C: representative immunoblots from n = 2 mice from 2 independent experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24376772), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VE-Cadherin Antibody by Western Blot Semiquantitative immunoblotting reveals differential Ad5ROBO4 reporter expression in tumor compared to liver.A. Immunoblot of EGFP, VE-cadherin, and beta -tubulin loading controls in tissue protein extracts from hCAR:Rag2−/− mice injected with either Ad5ROBO4, left three lanes, or Ad5CMV, right three lanes. B. and C. Densitometry analysis of Ad5ROBO4 vector EGFP expression normalized to either VE-cadherin or beta -tubulin. D. Densitometry of AdCMV vector EGFP expression. As AdCMV expression was hepatocyte specific, this blot was only normalized to beta -tubulin. A–D: Representative immunoblots from n = 4 mice injected with either Ad5ROBO 4 or Ad5CMV vectors. Li: liver, KO: kidney orthotopic tumor, SC: subcutaneous tumor. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24376772), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VE-Cadherin Antibody by Western Blot Endogenous ROBO4 upregulation despite lower vascular density in orthotopic and xenograft tumors.A. Immunofluorescence of the vascular endothelium in liver (upper panel) and 786-O human renal cell carcinoma (RCC) subcutaneous xenograft tumor (lower panel). B. Vascular area analysis of liver (Li), kidney orthotopic (KO) tumors, and subcutaneous (SC) xenograft tumors (n = 6 mice analyzed). C. Immunoblot of endogenous ROBO4 and the endothelial cell specific VE-cadherin from liver, kidney orthotopic and subcutaneous xenograft 786-O RCC tumors, and from the derivative 786-O cells grown in culture. D. Densitometry analysis of VE-cadherin/tubulin ratio from C mirrors the vascular area determination in B. E. Densitometry analysis of endogenous ROBO4 normalized to VE-cadherin expression reveals a 1.4- to 2-fold increase in SC and KO tumors compared to liver. C–E: Immunoblot and densitometry was repeated twice with two independent sets of protein extracts from two different tumor-bearing mice with essentially the same results. A. Magnification: 100X, Red: endomucin/CD31 antibody cocktail, Blue: DAPI. B. *p<0.05, one way ANOVA with Tukey's correction, mean ± SD. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24376772), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse Mouse VE-Cadherin Antibody by Western Blot Warfarin pretreatment enhances tumor Ad5ROBO4 vector expression.A. Multiorgan immunoblot of vehicle (left lanes) or warfarin (right lanes) pretreated Rag2−/− mice injected with 1.0×1011 vp of Ad5ROBO4. B. Densitometry of A revealed that vehicle pretreatment was associated with robust liver, detectable splenic, and trace to undetectable expression in all other sampled organs. Warfarin pretreatment produced a 2.5-fold increased splenic and a 3-fold decreased liver expression while all other organs still evidenced trace to undetectable expression. C. Immunoblot and densitometry of liver and tumor EGFP, VE-cadherin, and beta -tubulin expression in vehicle (left lanes) or warfarin (right lanes) from the same pretreated, Ad5ROBO4-injected mice as in A and B. EGFP densitometry normalized to VE-cadherin, revealed a 4.7-fold decrease in liver and 2-fold increase in increase KO and SC tumor expression produced by warfarin pretreatment. A–C: representative immunoblots from n = 2 mice from 2 independent experiments. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24376772), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
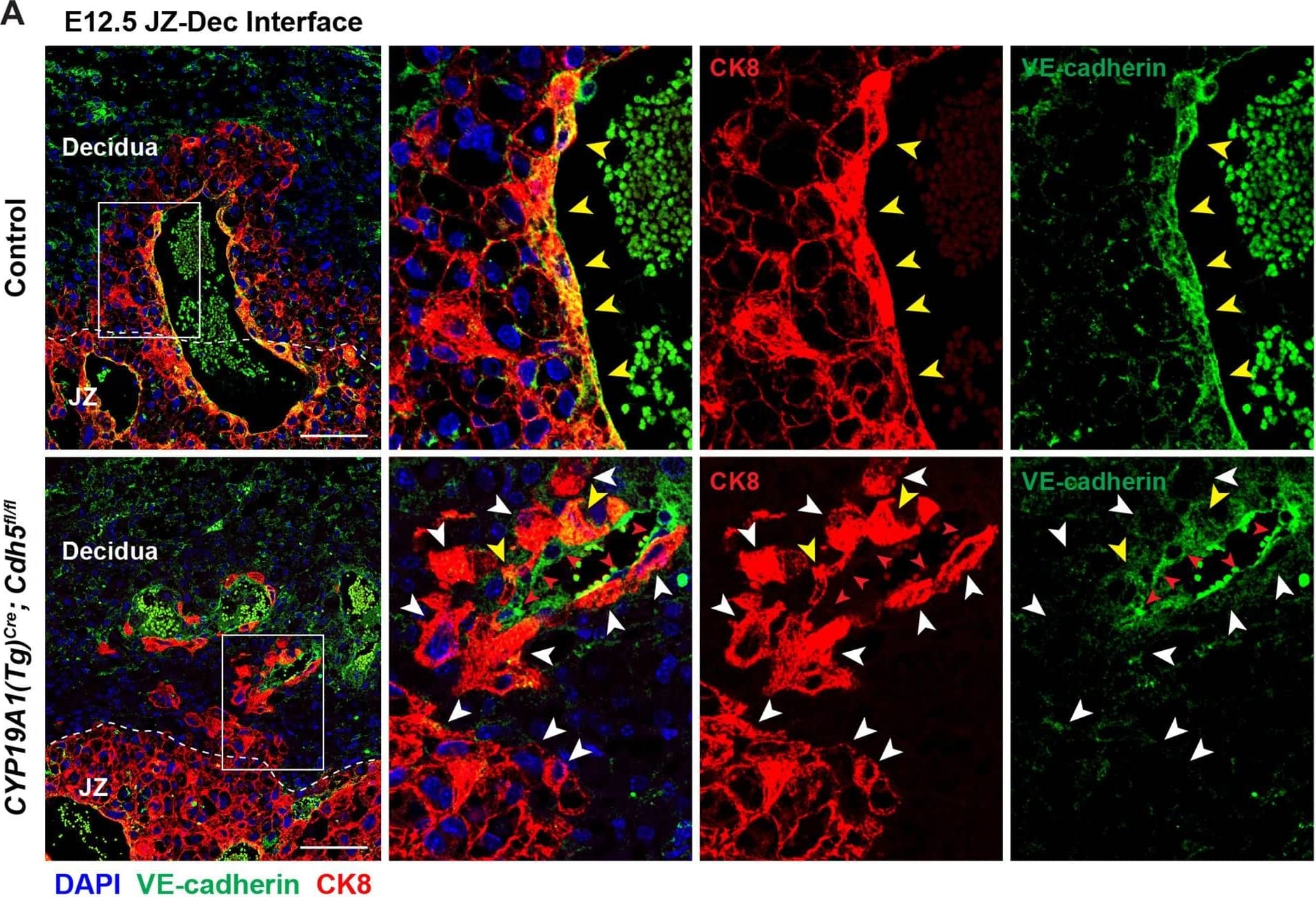
Detection of Mouse VE-Cadherin by Immunohistochemistry Deletion of VE-cadherin in CYP19A1(Tg)Cre; Cdh5fl/fl placentas.(A, B) Immunofluorescence staining and quantification of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl placentas for VE-cadherin (green) and CK8 (red). Yellow arrowheads indicate VE-cadherin+ trophoblasts, which predominantly line remodeled spiral arteries. White arrowheads indicate VE-cadherin- trophoblasts. Red arrowheads indicate spiral artery endothelial cells (ECs), which are VE-cadherin+ CK8-. VE-cadherin and CK8 colocalized area was divided by total CK8-positive area to determine the extent of VE-cadherin deletion. Note that there are a few VE-cadherin+ trophoblasts in CYP19A1(Tg)Cre; Cdh5fl/fl placentas, however VE-cadherin is deleted from the majority of trophoblasts. The layer of VE-cadherin+ CK8- cells (red arrowheads) in the CYP19A1(Tg)Cre; Cdh5fl/fl placentas are spiral artery endothelial cells that have not been displaced by trophoblasts. Dotted white lines demarcate the decidua and junctional zone (JZ). Positive signal in small, rounded cells in the lumen is the result of erythrocyte autofluorescence. Control n = 4, CYP19A1(Tg)Cre; Cdh5fl/fl n = 3. Scale bars = 100 μm. (C, D) E12.5 placental and embryo weights do not differ between Cre-negative (Cdh5fl/+ or Cdh5fl/fl) and Cre-positive heterozygous (CYP19A1(Tg)Cre; Cdh5fl/fl) controls. Statistical analysis was performed using two-tailed, unpaired Welch’s t-test. Data are shown as means ± SD.Figure 1—figure supplement 1—source data 1.Excel file containing quantification for VE-cadherin expression in trophoblasts, embryo weights, and placenta weights in Figure 1—figure supplement 1.Excel file containing quantification for VE-cadherin expression in trophoblasts, embryo weights, and placenta weights in Figure 1—figure supplement 1. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35486098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
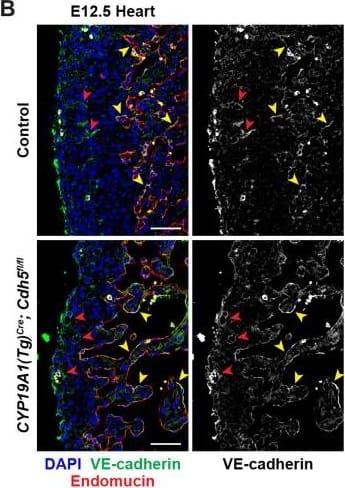
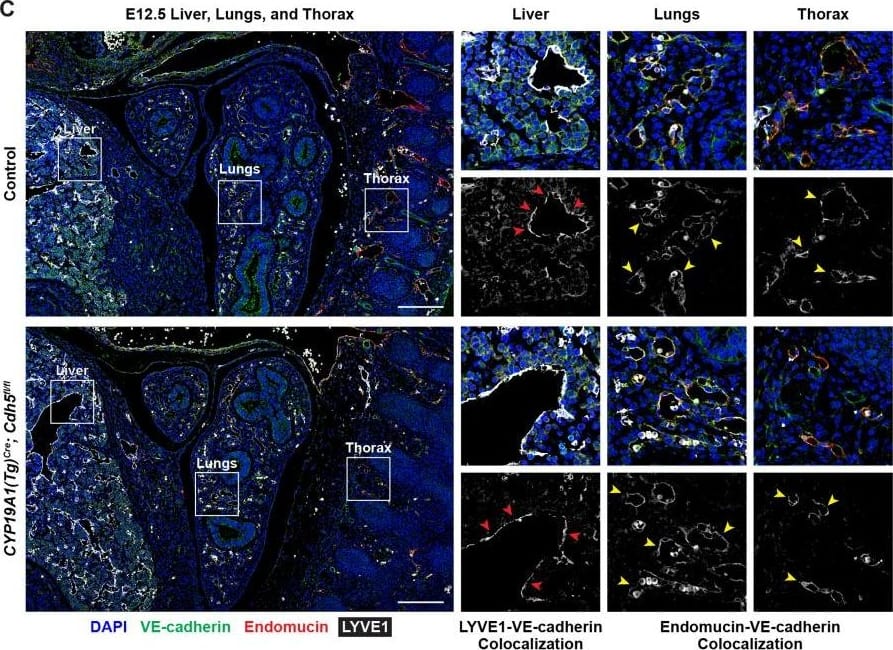
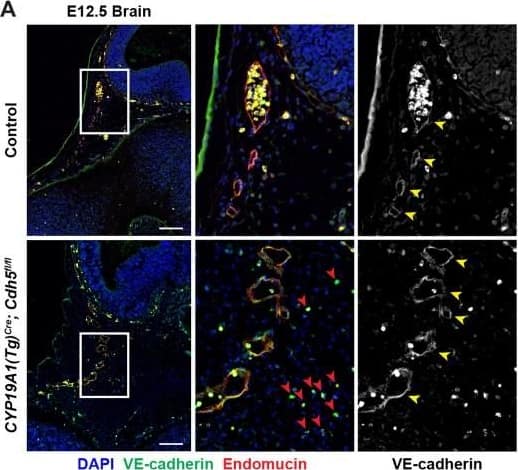
Detection of Mouse VE-Cadherin by Immunohistochemistry VE-cadherin expression is retained in the vasculature of affected organs in CYP19A1(Tg)Cre; Cdh5fl/fl embryos.(A) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl brains for VE-cadherin (green) and Endomucin (red) at sites of hemorrhage. Images in gray scale are VE-cadherin alone. Red arrowheads point to extravascular autofluorescent erythrocytes. Yellow arrowheads point to VE-cadherin+ vessels. Scale bars = 50 μm. (B) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green) and Endomucin (red). Images in gray scale are VE-cadherin alone. Red arrowheads point to VE-cadherin+ developing coronary vessels. Yellow arrowheads point to VE-cadherin+ endocardium. Scale bars = 50 μm. (C) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green), Endomucin (red), and LYVE1 (gray). Images in gray scale represent VE-cadherin pixels colocalized with either LYVE1 (liver) or Endomucin (lungs and thorax). Red arrowheads point to LYVE1+VE-cadherin+ liver sinusoidal vessels. Yellow arrowheads point to Endomucin+VE-cadherin+ lung and thoracic blood vessels. Scale bars = 200 μm. (D) Quantification of fold change in VE-cadherin mean fluorescence intensity in the brain, heart, liver, lungs, and thorax.Figure 1—figure supplement 3—source data 1.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35486098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry VE-cadherin expression is retained in the vasculature of affected organs in CYP19A1(Tg)Cre; Cdh5fl/fl embryos.(A) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl brains for VE-cadherin (green) and Endomucin (red) at sites of hemorrhage. Images in gray scale are VE-cadherin alone. Red arrowheads point to extravascular autofluorescent erythrocytes. Yellow arrowheads point to VE-cadherin+ vessels. Scale bars = 50 μm. (B) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green) and Endomucin (red). Images in gray scale are VE-cadherin alone. Red arrowheads point to VE-cadherin+ developing coronary vessels. Yellow arrowheads point to VE-cadherin+ endocardium. Scale bars = 50 μm. (C) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green), Endomucin (red), and LYVE1 (gray). Images in gray scale represent VE-cadherin pixels colocalized with either LYVE1 (liver) or Endomucin (lungs and thorax). Red arrowheads point to LYVE1+VE-cadherin+ liver sinusoidal vessels. Yellow arrowheads point to Endomucin+VE-cadherin+ lung and thoracic blood vessels. Scale bars = 200 μm. (D) Quantification of fold change in VE-cadherin mean fluorescence intensity in the brain, heart, liver, lungs, and thorax.Figure 1—figure supplement 3—source data 1.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35486098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
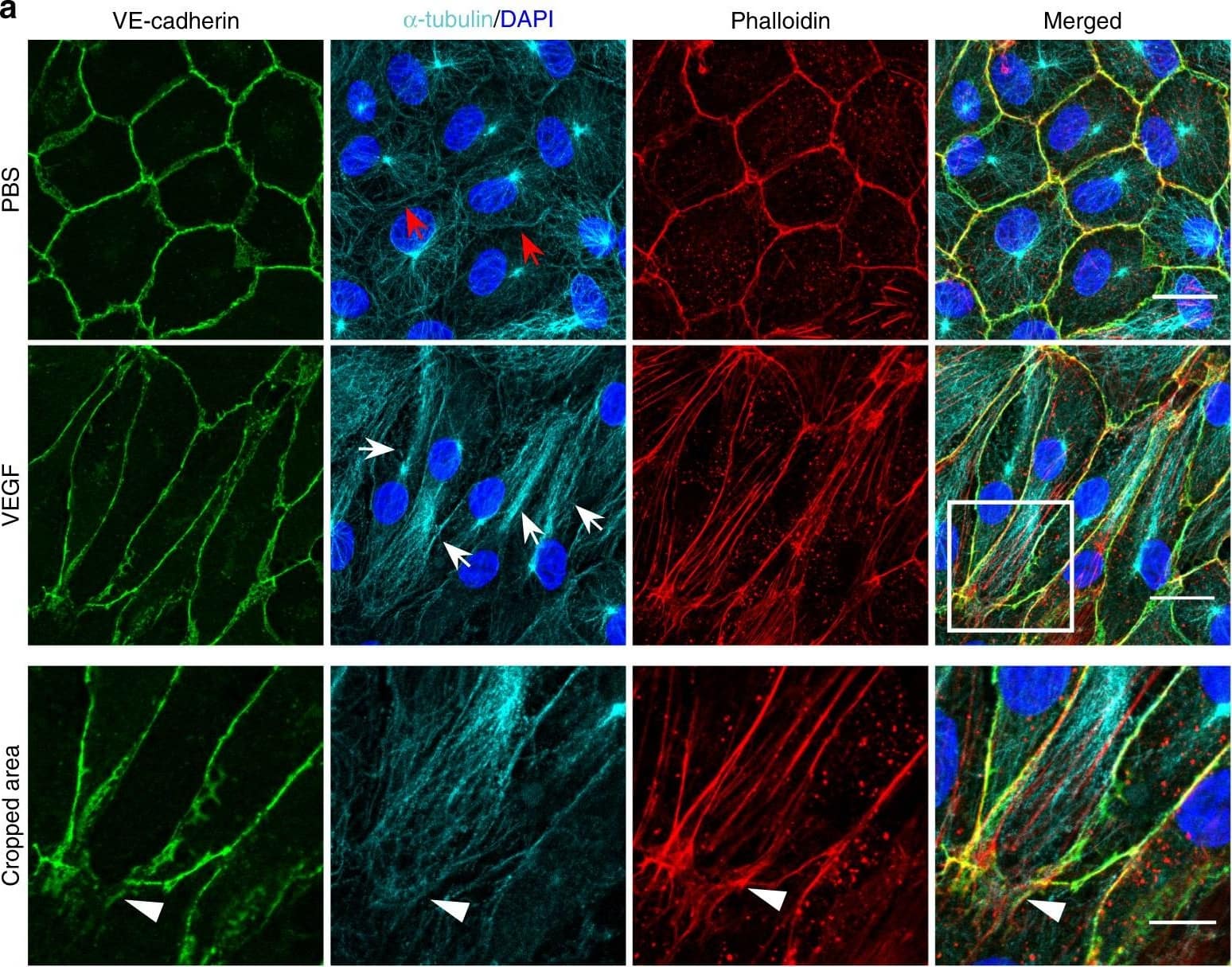
Detection of Mouse VE-Cadherin by Immunohistochemistry Microtubules (MT) are indispensable for VEGF-induced cell elongation. a Confluent HUVECs immune labelled for VE-cadherin, alpha -tubulin and Phalloidin-TRITC after VEGF treatment for 24 h or PBS for control, as indicated. Nuclei are stained blue with DAPI. LSM demonstrates MT in control cells evenly distributed throughout the cells, while a few MT are aligned in parallel with JAAF (red arrows). VEGF-induced elongated cells display MT running parallel to the longitudinal cell axis together with stress fibres, and MT are enriched at the leading edge (white arrows; for dynamics compare supplementary Movie 10) (Scale bar: 20 µm). The cropped area displays an interrupted VE-cadherin pattern, MT enrichment, and stress fibres at the cell poles (arrowheads; for dynamics compare Supplementary Movies 10 and 11) (scale bar: 10 µm). b Confluent HUVEC cultures treated with 50 ng ml−1 nocodazole for 4 h and subsequently labelled with VE-cadherin antibody and with Phallodin-TRITC for actin filaments. MT depolymerisation had less effect on the JAAF and VE-cadherin distribution (Scale bar: 20 µm). c–e Confluent HUVECs pre-treated with 50 ng ml−1 nocodazole for 30 min and then treated with VEGF for another 18 h. c Phase-contrast microscopy revealed that nocodazole inhibited VEGF-induced cell elongation (scale bar: 80 µm). Quantification of (d) cell velocity and (e) cell elongation using Fiji software (100 cells were analysed at t = 0, 100 cells at t = 9 h, 81 cells at t = 18 h for nocodazole+VEGF treatment; and 100 cells were analysed at each time point for VEGF treatment, unpaired student’s t test). noco: nocodazole. Representative results from three independent experiments are shown. Error bars indicate ± SEM Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry VE-cadherin expression is retained in the vasculature of affected organs in CYP19A1(Tg)Cre; Cdh5fl/fl embryos.(A) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl brains for VE-cadherin (green) and Endomucin (red) at sites of hemorrhage. Images in gray scale are VE-cadherin alone. Red arrowheads point to extravascular autofluorescent erythrocytes. Yellow arrowheads point to VE-cadherin+ vessels. Scale bars = 50 μm. (B) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green) and Endomucin (red). Images in gray scale are VE-cadherin alone. Red arrowheads point to VE-cadherin+ developing coronary vessels. Yellow arrowheads point to VE-cadherin+ endocardium. Scale bars = 50 μm. (C) Immunofluorescence staining of E12.5 Control and CYP19A1(Tg)Cre; Cdh5fl/fl hearts for VE-cadherin (green), Endomucin (red), and LYVE1 (gray). Images in gray scale represent VE-cadherin pixels colocalized with either LYVE1 (liver) or Endomucin (lungs and thorax). Red arrowheads point to LYVE1+VE-cadherin+ liver sinusoidal vessels. Yellow arrowheads point to Endomucin+VE-cadherin+ lung and thoracic blood vessels. Scale bars = 200 μm. (D) Quantification of fold change in VE-cadherin mean fluorescence intensity in the brain, heart, liver, lungs, and thorax.Figure 1—figure supplement 3—source data 1.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3.Excel file containing quantification for VE-cadherin expression in various organs in Figure 1—figure supplement 3. Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/35486098), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
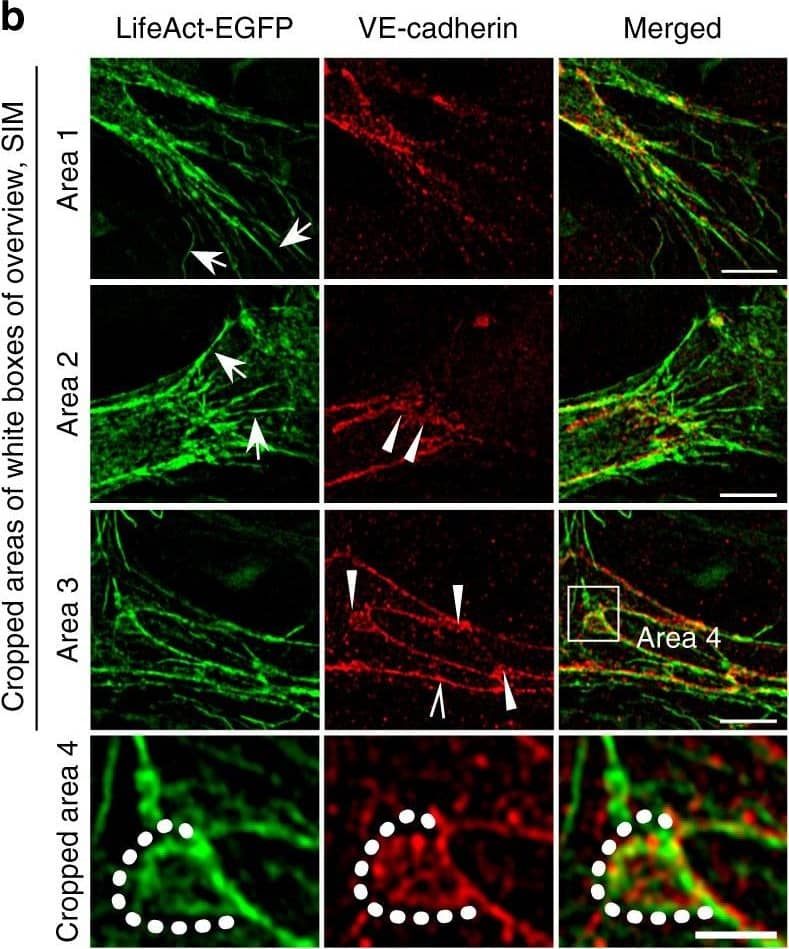
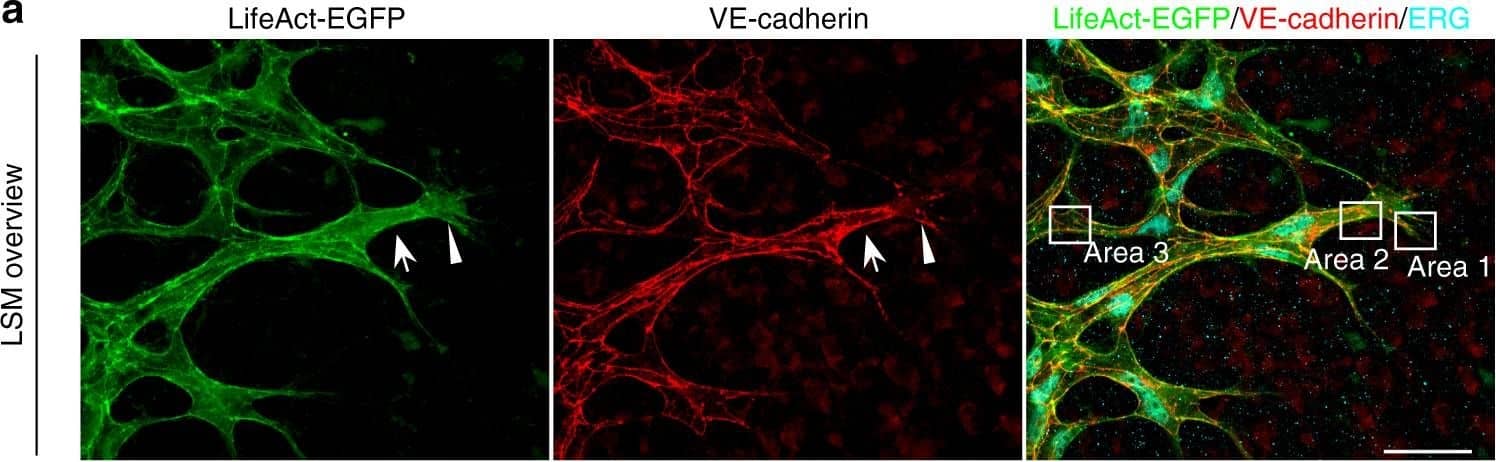
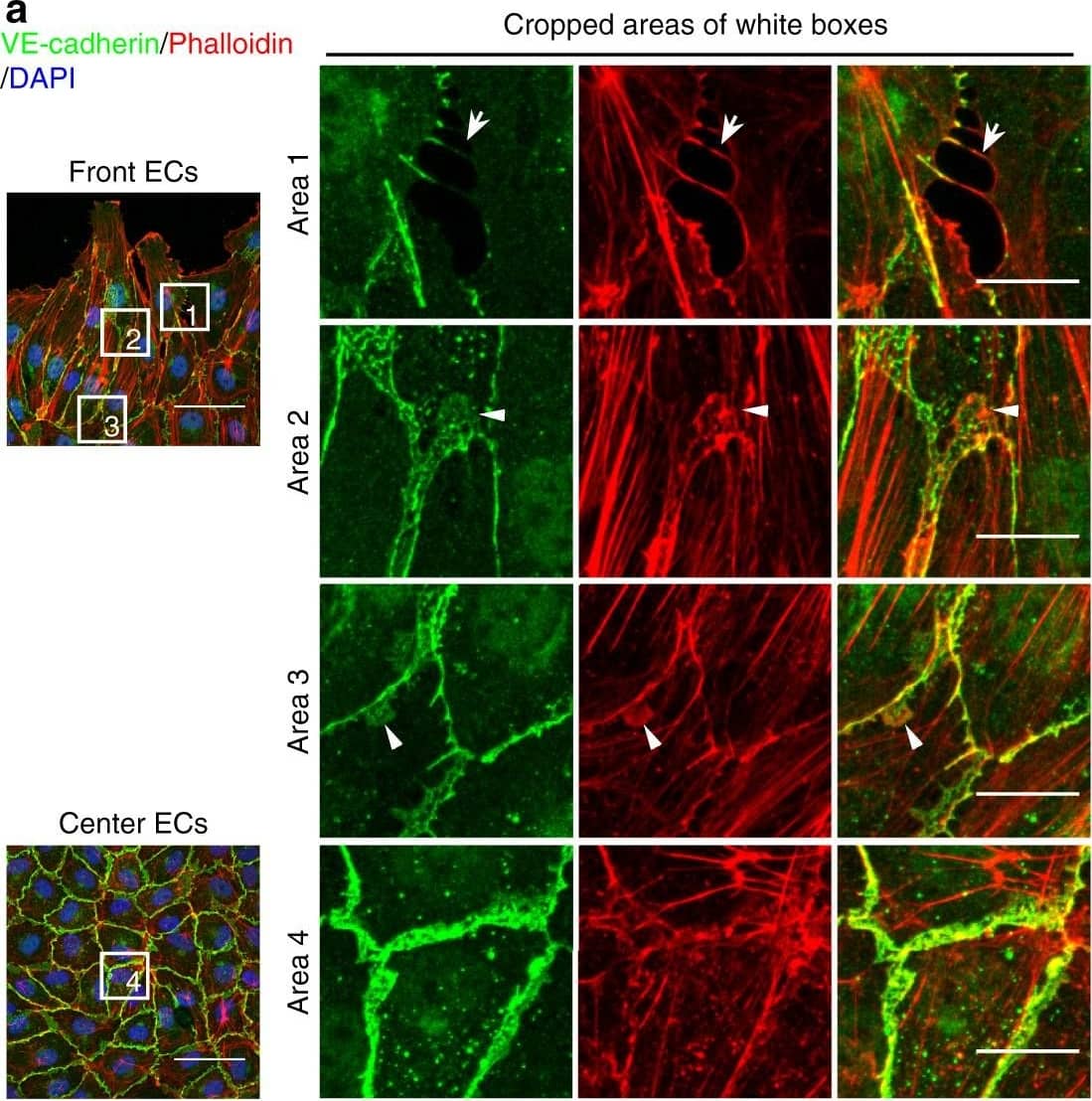
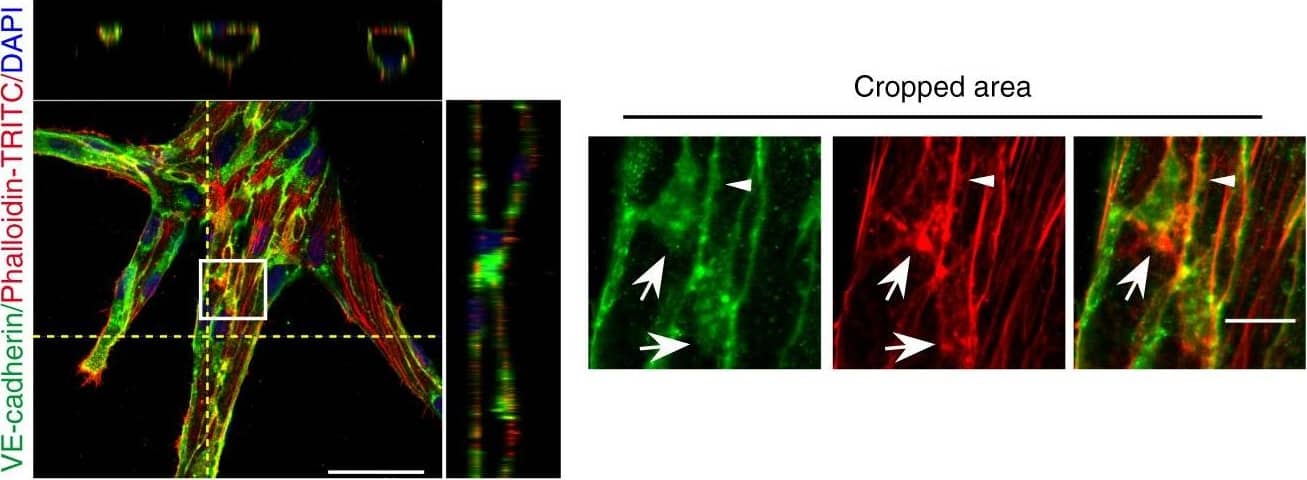
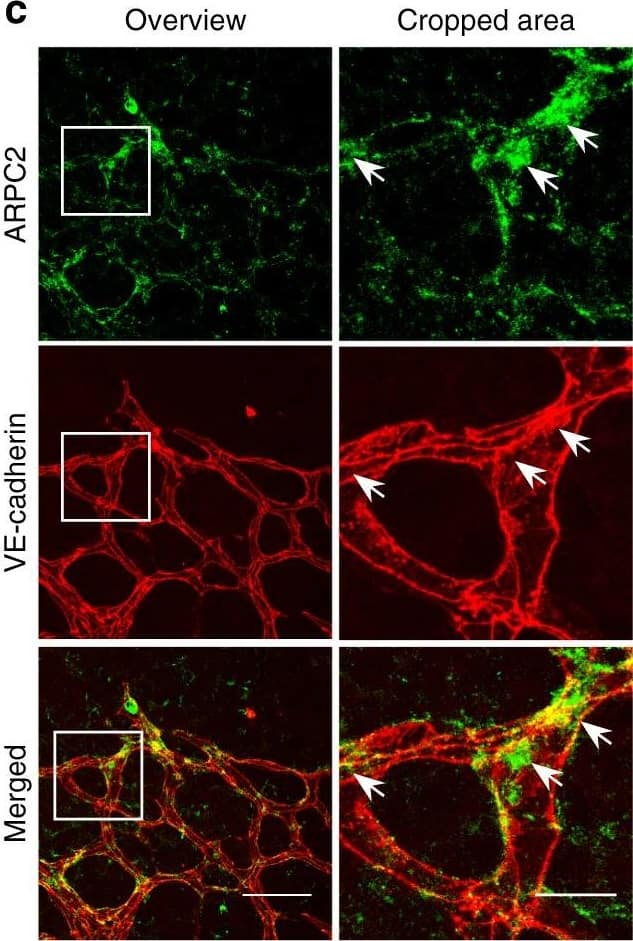
Detection of Mouse VE-Cadherin by Immunohistochemistry Polarized distribution of VE-cadherin and actin in sprouting ECs in developing mice retinas. a Laser scanning microscopy (LSM) showing an overview of the front area of a whole-mounted P6 transgenic mouse retina expressing LifeAct-EGFP additionally immune stained with anti-VE-cadherin and anti-ERG antibodies; nuclei were stained with anti-ERG. Tip cell (arrowheads) and adjacent stalk cell (arrows) are indicated. Scale bar: 40 µm. b High-resolution SIM of selected areas 1–3, as indicated in the right panel of a. Shown is one z-plane. (Area 1) a characteristic tip cell with large actin-based filopodia and a cytosolic spotted VE-cadherin pattern. (Area 2) A tip cell/stalk cell junction at the cell pole of elongated cells identifies terminating actin filaments (arrow) and an interrupted VE-cadherin pattern (arrowhead). (Area 3) a stalk cell/stalk cell connection. VE-cadherin plaques are indicated at the cell poles (arrowheads) and a linear VE-cadherin pattern (empty arrowhead) at lateral junctions; parallel actin filaments are also visible. Scale bar: 5 µm. (Area 4) The cropped area depicts an actin-positive JAIL (dotted line, LifeAct-EGFP) with VE-cadherin plaques (dotted line, VE-cadherin staining). Scale bar: 2 µm. c P7 rat retina immune labelled with ARPC2 and VE-cadherin show increased ARPC2 at the cell poles (arrows). Scale bars in the left panels and right panels represent 50 and 15 µm, respectively. d Scheme illustrates the iterative dynamics of VE-cadherin interruption and JAIL formation leading to VE-cadherin plaques in sprouting ECs. The VE-cadherin dynamics was particularly pronounced at the cell poles, while the lateral junctions showed moderate dynamics Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry Polarized distribution of VE-cadherin and actin in sprouting ECs in developing mice retinas. a Laser scanning microscopy (LSM) showing an overview of the front area of a whole-mounted P6 transgenic mouse retina expressing LifeAct-EGFP additionally immune stained with anti-VE-cadherin and anti-ERG antibodies; nuclei were stained with anti-ERG. Tip cell (arrowheads) and adjacent stalk cell (arrows) are indicated. Scale bar: 40 µm. b High-resolution SIM of selected areas 1–3, as indicated in the right panel of a. Shown is one z-plane. (Area 1) a characteristic tip cell with large actin-based filopodia and a cytosolic spotted VE-cadherin pattern. (Area 2) A tip cell/stalk cell junction at the cell pole of elongated cells identifies terminating actin filaments (arrow) and an interrupted VE-cadherin pattern (arrowhead). (Area 3) a stalk cell/stalk cell connection. VE-cadherin plaques are indicated at the cell poles (arrowheads) and a linear VE-cadherin pattern (empty arrowhead) at lateral junctions; parallel actin filaments are also visible. Scale bar: 5 µm. (Area 4) The cropped area depicts an actin-positive JAIL (dotted line, LifeAct-EGFP) with VE-cadherin plaques (dotted line, VE-cadherin staining). Scale bar: 2 µm. c P7 rat retina immune labelled with ARPC2 and VE-cadherin show increased ARPC2 at the cell poles (arrows). Scale bars in the left panels and right panels represent 50 and 15 µm, respectively. d Scheme illustrates the iterative dynamics of VE-cadherin interruption and JAIL formation leading to VE-cadherin plaques in sprouting ECs. The VE-cadherin dynamics was particularly pronounced at the cell poles, while the lateral junctions showed moderate dynamics Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry JAIL formation&cell migration are blocked by Rac inhibition&VE-cadherin overexpression in scratch assay. a–f HUVECs scratched, allowed to grow for 5 h,&labelled for VE-cadherin&Phalloidin-TRITC. a Overviews of the migrating front (left upper)¢re area (left lower). (Area 1) interrupted VE-cadherin that co-localise with filopodia-like actin filaments (arrows). (Area 2) Large JAIL (arrowheads) at cell pole. (Area 3) small JAIL (arrowheads) at lateral junctions. (Area 4) Polygonal cells in the centre area. Scale bars represent 60 µm&15 µm in the overview&cropped images respectively. b, c Comparison of cell perimeter&Rel-VEcad-C in front¢re ECs. Quantification of (d) JAIL number&(e) JAIL size in front (n = 115 cells)¢re cells (n = 186 cells). f JAIL size at cell poles&lateral junctions in front ECs (n = 115 cells). P value was determined by unpaired student’s t test. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry Polarized JAIL dynamics during sprouting angiogenesis in fibrin angiogenesis assay using EGFP-p20 expressing HUVECs.f Overview&Z-projections of vessel sprouts in fibrin angiogenesis assays 5 days after seeding; cells fixed&labelled with Phalloidin-TRITC&VE-cadherin. (cropped areas) JAIL are indicated at cell poles by the appearance of large VE-cadherin plaques (arrows) that co-localise with the actin network (arrows), whereas small VE-cadherin plaques appear at lateral junctions (arrowhead). Error bars represent ± SEM; scale bars indicate 50&10 µm in the overview&cropped areas, respectively Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse VE-Cadherin by Immunohistochemistry Polarized distribution of VE-cadherin and actin in sprouting ECs in developing mice retinas. a Laser scanning microscopy (LSM) showing an overview of the front area of a whole-mounted P6 transgenic mouse retina expressing LifeAct-EGFP additionally immune stained with anti-VE-cadherin and anti-ERG antibodies; nuclei were stained with anti-ERG. Tip cell (arrowheads) and adjacent stalk cell (arrows) are indicated. Scale bar: 40 µm. b High-resolution SIM of selected areas 1–3, as indicated in the right panel of a. Shown is one z-plane. (Area 1) a characteristic tip cell with large actin-based filopodia and a cytosolic spotted VE-cadherin pattern. (Area 2) A tip cell/stalk cell junction at the cell pole of elongated cells identifies terminating actin filaments (arrow) and an interrupted VE-cadherin pattern (arrowhead). (Area 3) a stalk cell/stalk cell connection. VE-cadherin plaques are indicated at the cell poles (arrowheads) and a linear VE-cadherin pattern (empty arrowhead) at lateral junctions; parallel actin filaments are also visible. Scale bar: 5 µm. (Area 4) The cropped area depicts an actin-positive JAIL (dotted line, LifeAct-EGFP) with VE-cadherin plaques (dotted line, VE-cadherin staining). Scale bar: 2 µm. c P7 rat retina immune labelled with ARPC2 and VE-cadherin show increased ARPC2 at the cell poles (arrows). Scale bars in the left panels and right panels represent 50 and 15 µm, respectively. d Scheme illustrates the iterative dynamics of VE-cadherin interruption and JAIL formation leading to VE-cadherin plaques in sprouting ECs. The VE-cadherin dynamics was particularly pronounced at the cell poles, while the lateral junctions showed moderate dynamics Image collected and cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/29263363), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VE-Cadherin
The cadherin (Ca++-dependent adherence) superfamily is a large group of membrane-associated glycoproteins that engage in homotypic, calcium-dependent, cell-cell adhesion events. The superfamily can be divided into at least five major subfamilies based on molecule gene structure, and/or extracellular (EC) and intracellular domains (1-4). Subfamilies include classical/type I, atypical/type II, and desmosomal-related cadherins (1-3). VE-Cadherin (vascular endothelial cadherin; also cadherin-5 and CD144) is a 125 kDa atypical/type II subfamily cadherin. Its subfamily classification is based principally on its genomic structure, as its physical structure is notably divergent from other type II subfamily members (2, 3). Mouse VE-Cadherin is synthesized as a 784 amino acid (aa) type I transmembrane (TM) preproprotein that contains a 24 aa signal peptide, a 21 aa prosequence, a 554 aa extracellular region (ECR), a 21 aa TM segment, and a 164 aa cytoplasmic domain (5, 6). The ECR contains five Ca++-binding cadherin domains that are approximately 105 aa in length. Cadherin domains are comprised of two beta ‑sheets that are oriented like bread in a sandwich. Although complex, the N-terminal cadherin domain mediates trans interactions, while the internal domains contribute to cis multimerizations (7). Mouse VE-Cadherin ECR is 92%, 77%, and 73% aa identical to rat, human and porcine VE-Cadherin ECR, respectively. VE-Cadherin is involved in the maintenance of endothelial permeability. In this regard, VE-Cadherin does not initiate new blood vessel formation; it maintains it once formed. Thus, when VE‑Cadherin is downregulated, cells part and permeability increases (8). Notably, VEGF is known to promote vascular leakage, and apparently does so by inducing a beta ‑arrestin-dependent endocytosis of VE-Cadherin (9). Part of this effect may be mediated by VE‑Cadherin itself which is reported to increase the membrane half-life of VEGF R2 (10). VE-Cadherin acts homotypically at sites of zonula adherens. On each expressing cell, it is proposed that VE-Cadherin first forms a trimer, which then dimerizes with a trimeric counterpart in-trans. Alternatively, two cis-dimers could act in-trans to generate homotypic binding (11). In addition to cell adhesion, VE‑Cadherin also is reported to mediate TGF-beta receptor assembly. When clustered, VE‑Cadherin enhances T beta RII/T beta RI assembly into an active receptor complex on endothelial cells (12). VE-Cadherin is expressed on endothelial cells, trophoblast cells, endothelial progenitor cells and embryonic hematopoietic cells (5, 8, 13, 14).
- Patel, S.D. et al. (2007) Curr. Opin. Struct. Biol. 13:690.
- Vestweber, D. (2008) Arterioscler. Thromb. Vasc. Biol. 28:223.
- Vincent, P.A. et al. (2004) Am. J. Physiol. Cell. Physiol. 286:C987.
- Cavallaro, U. et al. (2006) Exp. Cell Res. 312:659.
- Breier, G. et al. (1996) Blood 87:630.
- Huber, P. et al. (1996) Genomics 32:21.
- Pokutta, S. and W.I. Weis (2007) Annu. Rev. Cell Dev. Biol. 23:237.
- Crosby, C.V. et al. (2005) Blood 105:2771.
- Gavard, J. and J.S. Gutkind (2006) Nat. Cell Biol. 8:1223.
- Calera, M.R. et al. (2004) Exp. Cell Res. 300:248.
- Hewat, E.A. et al. (2007) J. Mol. Biol. 365:744.
- Rudini, N. et al. (2008) EMBO J. 27:993.
- Kogata, N. et al. (2006) Circ. Res. 98:897.
- Ema, M. et al. (2006) Blood 108:4018.
Product Datasheets
Citations for Mouse VE-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
119
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Annexin A2 supports pulmonary microvascular integrity by linking vascular endothelial cadherin and protein tyrosine phosphatases
Authors: Luo M, Flood EC, Almeida D et al.
J. Exp. Med.
-
Foxc1 and Foxc2 deletion causes abnormal lymphangiogenesis and correlates with ERK hyperactivation
J Clin Invest, 2016-05-23;0(0):.
-
Sphingosine 1-Phosphate Receptor Signaling Establishes AP-1 Gradients to Allow for Retinal Endothelial Cell Specialization
Authors: Yanagida K, Engelbrecht E, Niaudet C et al.
Dev. Cell
-
Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery
Authors: Xue, L;Zhao, G;Gong, N;Han, X;Shepherd, SJ;Xiong, X;Xiao, Z;Palanki, R;Xu, J;Swingle, KL;Warzecha, CC;El-Mayta, R;Chowdhary, V;Yoon, IC;Xu, J;Cui, J;Shi, Y;Alameh, MG;Wang, K;Wang, L;Pochan, DJ;Weissman, D;Vaughan, AE;Wilson, JM;Mitchell, MJ;
Nature nanotechnology
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Apelin modulates inflammation and leukocyte recruitment in experimental autoimmune encephalomyelitis
Authors: Park, H;Song, J;Jeong, HW;Grönloh, MLB;Koh, BI;Bovay, E;Kim, KP;Klotz, L;Thistlethwaite, PA;van Buul, JD;Sorokin, L;Adams, RH;
Nature communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
?1 integrins play a critical role maintaining vascular integrity in the hypoxic spinal cord, particularly in white matter
Authors: Halder, SK;Sapkota, A;Milner, R;
Acta neuropathologica communications
Species: Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
An EPHB4-RASA1 signaling complex inhibits shear stress-induced Ras-MAPK activation in lymphatic endothelial cells to promote the development of lymphatic vessel valves
Authors: Chen, D;Wiggins, D;Sevick, EM;Davis, MJ;King, PD;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Identification of CD133+ intercellsomes in intercellular communication to offset intracellular signal deficit
Authors: Kaneko, K;Liang, Y;Liu, Q;Zhang, S;Scheiter, A;Song, D;Feng, GS;
eLife
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Spatial-temporal proliferation of hepatocytes during pregnancy revealed by genetic lineage tracing
Authors: He, S;Guo, Z;Zhou, M;Wang, H;Zhang, Z;Shi, M;Li, X;Yang, X;He, L;
Cell stem cell
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
Alk1 acts in non-endothelial VE-cadherin+ perineurial cells to maintain nerve branching during hair homeostasis
Authors: Chovatiya, G;Li, KN;Li, J;Ghuwalewala, S;Tumbar, T;
Nature communications
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
FYN regulates aqueous humor outflow and IOP through the phosphorylation of VE-cadherin
Authors: Kizhatil, K;Clark, G;Sunderland, D;Bhandari, A;Horbal, L;Balasubramanian, R;John, S;
bioRxiv : the preprint server for biology
Species: Transgenic Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Temporal Progression of Aortic Valve Pathogenesis in a Mouse Model of Osteogenesis Imperfecta
Authors: Thatcher, K;Mattern, CR;Chaparro, D;Goveas, V;McDermott, MR;Fulton, J;Hutcheson, JD;Hoffmann, BR;Lincoln, J;
Journal of cardiovascular development and disease
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Prolonged hypoxia alleviates prolyl hydroxylation-mediated suppression of RIPK1 to promote necroptosis and inflammation
Authors: Zhang, T;Xu, D;Liu, J;Wang, M;Duan, LJ;Liu, M;Meng, H;Zhuang, Y;Wang, H;Wang, Y;Lv, M;Zhang, Z;Hu, J;Shi, L;Guo, R;Xie, X;Liu, H;Erickson, E;Wang, Y;Yu, W;Dang, F;Guan, D;Jiang, C;Dai, X;Inuzuka, H;Yan, P;Wang, J;Babuta, M;Lian, G;Tu, Z;Miao, J;Szabo, G;Fong, GH;Karnoub, AE;Lee, YR;Pan, L;Kaelin, WG;Yuan, J;Wei, W;
Nature cell biology
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Partial Mural Cell Ablation Disrupts Coronary Vasculature Integrity and Induces Systolic Dysfunction
Authors: Cornuault, L;Hérion, FX;Bourguignon, C;Rouault, P;Foussard, N;Alzieu, P;Chapouly, C;Gadeau, AP;Couffinhal, T;Renault, MA;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
Cytarabine induces cachexia with lipid malabsorption via zippering the junctions of lacteal in murine small intestine
Authors: Mi-Rae Park, Hye-Jin Lee, Hye-Min Jang, Nam Hoon Kim, Jun-Seok Lee, Yong Taek Jeong et al.
Journal of Lipid Research
-
EZH2 controls epicardial cell migration during heart development
Authors: Jiang H, Bai L, Song S et al.
Life science alliance
-
Therapeutic activation of endothelial sphingosine‐1‐phosphate receptor 1 by chaperone‐bound S1P suppresses proliferative retinal neovascularization
Authors: Colin Niaudet, Bongnam Jung, Andrew Kuo, Steven Swendeman, Edward Bull, Takahiro Seno et al.
EMBO Molecular Medicine
-
Osteoprogenitor-GMP crosstalk underpins solid tumor-induced systemic immunosuppression and persists after tumor removal
Authors: Hao, X;Shen, Y;Chen, N;Zhang, W;Valverde, E;Wu, L;Chan, HL;Xu, Z;Yu, L;Gao, Y;Bado, I;Michie, LN;Rivas, CH;Dominguez, LB;Aguirre, S;Pingel, BC;Wu, YH;Liu, F;Ding, Y;Edwards, DG;Liu, J;Alexander, A;Ueno, NT;Hsueh, PR;Tu, CY;Liu, LC;Chen, SH;Hung, MC;Lim, B;Zhang, XH;
Cell stem cell
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Endothelial deletion of PTBP1 disrupts ventricular chamber development
Authors: H Liu, R Duan, X He, J Qi, T Xing, Y Wu, L Zhou, L Wang, Y Shao, F Zhang, H Zhou, X Gu, B Lin, Y Liu, Y Wang, Y Liu, L Li, D Liang, YH Chen
Nature Communications, 2023-03-31;14(1):1796.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Trans-differentiation of trophoblast stem cells: implications in placental biology
Authors: Madhurima Paul, Shreeta Chakraborty, Safirul Islam, Rupasri Ain
Life Science Alliance
-
IFNgamma blockade in capillary leak site improves tumour chemotherapy by inhibiting lactate-induced endocytosis of vascular endothelial-cadherins
Authors: R Wang, C Ni, X Lou, L Zhang, L Wang, X Yao, X Duan, J Wan, P Li, Z Qin
International journal of biological sciences, 2023-02-27;19(5):1490-1508.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC, Western Blot, ICC -
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone
Authors: E Lee, EA Lee, E Kong, H Chon, M Llaiqui-Co, CH Park, BY Park, NR Kang, JS Yoo, HS Lee, HS Kim, SH Park, SW Choi, D Vestweber, JH Lee, P Kim, WS Lee, I Kim
Experimental & Molecular Medicine, 2023-02-24;55(2):470-484.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Shear stress control of vascular leaks and atheromas through Tie2 activation by VE-PTP sequestration
Authors: K Shirakura, P Baluk, AF Nottebaum, U Ipe, KG Peters, DM McDonald, D Vestweber
Embo Molecular Medicine, 2023-02-06;0(0):e16128.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone metastasis initiation is coupled with bone remodeling through osteogenic differentiation of NG2+ cells
Authors: W Zhang, Z Xu, X Hao, T He, J Li, Y Shen, K Liu, Y Gao, J Liu, D Edwards, AM Muscarella, L Wu, L Yu, L Xu, X Chen, YH Wu, IL Bado, Y Ding, S Aguirre, H Wang, Z Gugala, RL Satcher, ST Wong, XH Zhang
Cancer Discovery, 2023-02-06;0(0):.
Species: Transgenic Mouse
Sample Types: Whole Tissue
Applications: IHC -
A Prox1 enhancer represses haematopoiesis in the lymphatic vasculature
Authors: J Kazenwadel, P Venugopal, A Oszmiana, J Toubia, L Arriola-Ma, V Panara, SG Piltz, C Brown, W Ma, AW Schreiber, K Koltowska, S Taoudi, PQ Thomas, HS Scott, NL Harvey
Nature, 2023-01-25;614(7947):343-348.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Deficiency of endothelial FGFR1 alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice
Authors: Yanrong Long, Hongbin Chen, Junchao Deng, Junjie Ning, Pengbo Yang, Lina Qiao et al.
Frontiers in Pharmacology
-
Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts
Authors: F Baganha, TJ Sluiter, RCM de Jong, LA van Alst, HAB Peters, JW Jukema, M Delibegovi, K Pettersson, PHA Quax, MR de Vries
International Journal of Molecular Sciences, 2022-11-07;23(21):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
MASP-2 and MASP-3 inhibitors block complement activation, inflammation, and microvascular stasis in a murine model of vaso-occlusion in sickle cell disease
Authors: John D. Belcher, Julia Nguyen, Chunsheng Chen, Fuad Abdulla, Ruan Conglin, Zalaya K. Ivy et al.
Translational Research
-
Endothelial deletion of TBK1 contributes to BRB dysfunction via CXCR4 phosphorylation suppression
Authors: Bowen Zhao, Yueqi Ni, Hong Zhang, Yin Zhao, Lu Li
Cell Death Discovery
-
Syndecan-2 selectively regulates VEGF-induced vascular permeability
Authors: F. Corti, E. Ristori, F. Rivera-Molina, D. Toomre, J. Zhang, J. Mihailovic et al.
Nature Cardiovascular Research
-
p73 is required for vessel integrity controlling endothelial junctional dynamics through Angiomotin
Authors: Laura Maeso-Alonso, Hugo Alonso-Olivares, Nicole Martínez-García, Lorena López-Ferreras, Javier Villoch-Fernández, Laura Puente-Santamaría et al.
Cellular and Molecular Life Sciences
-
SARS-CoV-2 disrupts respiratory vascular barriers by suppressing Claudin-5 expression
Authors: R Hashimoto, J Takahashi, K Shirakura, R Funatsu, K Kosugi, S Deguchi, M Yamamoto, Y Tsunoda, M Morita, K Muraoka, M Tanaka, T Kanbara, S Tanaka, S Tamiya, N Tokunoh, A Kawai, M Ikawa, C Ono, K Tachibana, M Kondoh, M Obana, Y Matsuura, A Ohsumi, T Noda, T Yamamoto, Y Yoshioka, YS Torisawa, H Date, Y Fujio, M Nagao, K Takayama, Y Okada
Science Advances, 2022-09-21;8(38):eabo6783.
Species: Human, Mouse
Sample Types: Cell Lysates, Tissue Homogenates
Applications: Western Blot -
Network pharmacology and experimental analysis to reveal the mechanism of Dan-Shen-Yin against endothelial to mesenchymal transition in atherosclerosis
Authors: Mengyun Hong, Yubiao Wu, Haiyi Zhang, Jinchao Gu, Juanjuan Chen, Yancheng Guan et al.
Frontiers in Pharmacology
-
Claudin5 protects the peripheral endothelial barrier in an organ and vessel-type-specific manner
Authors: Mark Richards, Emmanuel Nwadozi, Sagnik Pal, Pernilla Martinsson, Mika Kaakinen, Marleen Gloger et al.
eLife
-
Identification and implication of tissue-enriched ligands in epithelial-endothelial crosstalk during pancreas development
Authors: M Moulis, SVM Runser, L Glorieux, N Dauguet, C Vanderaa, L Gatto, D Tyteca, P Henriet, FM Spagnoli, D Iber, CE Pierreux
Scientific Reports, 2022-07-21;12(1):12498.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Caspase-8 in endothelial cells maintains gut homeostasis and prevents small bowel inflammation in mice
Authors: N Tisch, C Mogler, A Stojanovic, R Luck, EA Korhonen, A Ellerkmann, H Adler, M Singhal, G Schermann, L Erkert, JV Patankar, A Karakatsan, AL Scherr, Y Fuchs, A Cerwenka, S Wirtz, BC Köhler, HG Augustin, C Becker, T Schmidt, C Ruiz de Al
Embo Molecular Medicine, 2022-05-02;0(0):e14121.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Structural and Functional Changes in Aged Skin Lymphatic Vessels
Authors: Raghu P. Kataru, Hyeung Ju Park, Jinyeon Shin, Jung Eun Baik, Ananta Sarker, Stav Brown et al.
Frontiers in Aging
-
The amyloid peptide beta disrupts intercellular junctions and increases endothelial permeability in a NADPH oxidase 1-dependent manner
Authors: A Tarafdar, N Wolska, C Krisp, H Schlüter, G Pula
Redox Biology, 2022-03-25;52(0):102287.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC/IF -
Engineering a niche supporting hematopoietic stem cell development using integrated single-cell transcriptomics
Authors: Brandon Hadland, Barbara Varnum-Finney, Stacey Dozono, Tessa Dignum, Cynthia Nourigat-McKay, Adam M. Heck et al.
Nature Communications
-
Force-induced changes of alpha-catenin conformation stabilize vascular junctions independent of vinculin
Authors: CN Duong, R Brückner, M Schmitt, AF Nottebaum, L Braun, MM Zu Brickwe, U Ipe, H Vom Bruch, HR Schöler, G Trapani, B Trappmann, MP Ebrahimkut, S Huveneers, J Rooij, N Ishiyama, M Ikura, D Vestweber
Journal of Cell Science, 2021-12-22;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Tgf?1-Cthrc1 Signaling Plays an Important Role in the Short-Term Reparative Response to Heart Valve Endothelial Injury
Authors: Nordquist EM, Dutta P, Kodigepalli KM et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Low-flow intussusception and metastable VEGFR2 signaling launch angiogenesis in ischemic muscle
Authors: JM Arpino, H Yin, EK Prescott, SCR Staples, Z Nong, F Li, J Chevalier, B Balint, C O'Neil, R Mortuza, S Milkovich, JJ Lee, D Lorusso, M Sandig, DW Hamilton, DW Holdsworth, TL Poepping, CG Ellis, JG Pickering
Science Advances, 2021-11-26;7(48):eabg9509.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lef1 restricts ectopic crypt formation and tumor cell growth in intestinal adenomas
Authors: S Heino, S Fang, M Lähde, J Högström, S Nassiri, A Campbell, D Flanagan, A Raven, M Hodder, N Nasreddin, HH Xue, M Delorenzi, S Leedham, TV Petrova, O Sansom, K Alitalo
Science Advances, 2021-11-17;7(47):eabj0512.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Full-length Dhh and N-terminal Shh act as competitive antagonists to regulate angiogenesis and vascular permeability
Authors: Pierre-Louis Hollier, Candice Chapouly, Aissata Diop, Sarah Guimbal, Lauriane Cornuault, Alain-Pierre Gadeau et al.
Cardiovascular Research
-
Imbalanced Activation of Wnt-/ beta -Catenin-Signaling in Liver Endothelium Alters Normal Sinusoidal Differentiation
Authors: Philipp-Sebastian Koch, Kajetan Sandorski, Joschka Heil, Christian D. Schmid, Sina W. Kürschner, Johannes Hoffmann et al.
Frontiers in Physiology
-
Fetal hematopoietic stem cell homing is controlled by VEGF regulating the integrity and oxidative status of the stromal-vascular bone marrow niches
Authors: M Mesnieres, AM Böhm, N Peredo, D Trompet, R Valle-Tenn, M Bajaj, N Corthout, E Nefyodova, R Cardoen, P Baatsen, S Munck, A Nagy, JJ Haigh, S Khurana, CM Verfaillie, C Maes
Cell Reports, 2021-08-24;36(8):109618.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lymphatic-specific intracellular modulation of receptor tyrosine kinase signaling improves lymphatic growth and function
Authors: Raghu P. Kataru, Jung Eun Baik, Hyeung Ju Park, Catherine L. Ly, Jinyeon Shin, Noa Schwartz et al.
Science Signaling
-
Atorvastatin pleiotropically decreases intraplaque angiogenesis and intraplaque haemorrhage by inhibiting ANGPT2 release and VE-Cadherin internalization
Authors: Fabiana Baganha, Rob C. M. de Jong, Erna A. Peters, Wietske Voorham, J. Wouter Jukema, Mirela Delibegovic et al.
Angiogenesis
-
Aminophylline modulates the permeability of endothelial cells via the Slit2‑Robo4 pathway in lipopolysaccharide‑induced inflammation
Authors: Qin Chen, Xiaoming Zhou, Ruonan Hou, Zhiliang Zhou, Zhiyi Wang, Ying Chen et al.
Experimental and Therapeutic Medicine
-
Protease nexin-1 deficiency increases mouse hindlimb neovascularisation following ischemia and accelerates femoral artery perfusion
Authors: S Selbonne, C Madjene, B Salmon, Y Boulaftali, MC Bouton, V Arocas
Scientific Reports, 2021-06-28;11(1):13412.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Intra-vessel heterogeneity establishes enhanced sites of macromolecular leakage downstream of laminin &alpha5
Authors: M Richards, S Pal, E Sjöberg, P Martinsson, L Venkatrama, L Claesson-W
Cell Reports, 2021-06-22;35(12):109268.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
eNOS-induced vascular barrier disruption in retinopathy by c-Src activation and tyrosine phosphorylation of VE-cadherin
Authors: Takeshi Ninchoji, Dominic T Love, Ross O Smith, Marie Hedlund, Dietmar Vestweber, William C Sessa et al.
eLife
-
The bone microenvironment invigorates metastatic seeds for further dissemination
Authors: Weijie Zhang, Igor L. Bado, Jingyuan Hu, Ying-Wooi Wan, Ling Wu, Hai Wang et al.
Cell
-
Paladin is a phosphoinositide phosphatase regulating endosomal VEGFR2 signalling and angiogenesis
Authors: Anja Nitzsche, Riikka Pietilä, Dominic T Love, Chiara Testini, Takeshi Ninchoji, Ross O Smith et al.
EMBO reports
-
CD112 Regulates Angiogenesis and T Cell Entry into the Spleen
Authors: E Russo, P Runge, NH Jahromi, H Naboth, A Landtwing, R Montecchi, N Leicht, MC Hunter, Y Takai, C Halin
Cells, 2021-01-15;10(1):.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging
Authors: Nicholas E. Propson, Ethan R. Roy, Alexandra Litvinchuk, Jörg Köhl, Hui Zheng
Journal of Clinical Investigation
-
A Type 2 Deiodinase-Dependent Increase in Vegfa Mediates Myoblast-Endothelial Cell Crosstalk During Skeletal Muscle Regeneration
Authors: Xingxing An, Ashley Ogawa-Wong, Colleen Carmody, Raffaele Ambrosio, Annunziata Gaetana Cicatiello, Cristina Luongo et al.
Thyroid
-
Desert Hedgehog-Driven Endothelium Integrity Is Enhanced by Gas1 (Growth Arrest-Specific 1) but Negatively Regulated by Cdon (Cell Adhesion Molecule-Related/Downregulated by Oncogenes)
Authors: Candice Chapouly, Pierre-Louis Hollier, Sarah Guimbal, Lauriane Cornuault, Alain-Pierre Gadeau, Marie-Ange Renault
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Regeneration of the pulmonary vascular endothelium after viral pneumonia requires COUP-TF2
Authors: Gan Zhao, Aaron I. Weiner, Katherine M. Neupauer, Maria Fernanda de Mello Costa, Gargi Palashikar, Stephanie Adams-Tzivelekidis et al.
Science Advances
-
Deficiency of peroxiredoxin 2 exacerbates angiotensin II-induced abdominal aortic aneurysm
Authors: SJ Jeong, MJ Cho, NY Ko, S Kim, IH Jung, JK Min, SH Lee, JG Park, GT Oh
Exp. Mol. Med., 2020-09-14;0(0):.
Species: Mouse
Sample Types: Cell Lysates
Applications: Western Blot -
Simulating flow induced migration in vascular remodelling
Authors: A Tabibian, S Ghaffari, DA Vargas, H Van Ooster, EAV Jones
PLoS Comput. Biol., 2020-08-21;16(8):e1007874.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Distinct fibroblast subsets regulate lacteal integrity through YAP/TAZ-induced VEGF-C in intestinal villi
Authors: SP Hong, MJ Yang, H Cho, I Park, H Bae, K Choe, SH Suh, RH Adams, K Alitalo, D Lim, GY Koh
Nat Commun, 2020-08-14;11(1):4102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
An autophagic deficit in the uterine vessel microenvironment provokes hyperpermeability through deregulated VEGFA, NOS1, and CTNNB1
Authors: B Lee, H Shin, JE Oh, J Park, M Park, SC Yang, JH Jun, SH Hong, H Song, HJ Lim
Autophagy, 2020-06-17;0(0):1-18.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation
Authors: Pieter R Norden, Amélie Sabine, Ying Wang, Cansaran Saygili Demir, Ting Liu, Tatiana V Petrova et al.
eLife
-
Atypical cadherin Fat4 orchestrates lymphatic endothelial cell polarity in response to flow
Authors: KL Betterman, DL Sutton, GA Secker, J Kazenwadel, A Oszmiana, L Lim, N Miura, L Sorokin, BM Hogan, ML Kahn, H McNeill, NL Harvey
J. Clin. Invest., 2020-06-01;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Canonical signaling by TGF family members in mesenchymal stromal cells is dispensable for hematopoietic niche maintenance under basal and stress conditions
Authors: JR Krambs, G Abou Ezzi, JC Yao, DC Link
PLoS ONE, 2020-05-29;15(5):e0233751.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Using apelin-based synthetic Notch receptors to detect angiogenesis and treat solid tumors
Authors: Z Wang, F Wang, J Zhong, T Zhu, Y Zheng, T Zhao, Q Xie, F Ma, R Li, Q Tang, F Xu, X Tian, J Zhu
Nat Commun, 2020-05-01;11(1):2163.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
c-Src controls stability of sprouting blood vessels in the developing retina independently of cell-cell adhesion through focal adhesion assembly
Authors: Lilian Schimmel, Daisuke Fukuhara, Mark Richards, Yi Jin, Patricia Essebier, Emmanuelle Frampton et al.
Development
-
Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta
Authors: Eric Engelbrecht, Michel V Levesque, Liqun He, Michael Vanlandewijck, Anja Nitzsche, Hira Niazi et al.
eLife
-
Interference With ESAM (Endothelial Cell-Selective Adhesion Molecule) Plus Vascular Endothelial-Cadherin Causes Immediate Lethality and Lung-Specific Blood Coagulation
Authors: Cao Nguyen Duong, Astrid F. Nottebaum, Stefan Butz, Stefan Volkery, Dagmar Zeuschner, Martin Stehling et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
EphA2 contributes to disruption of the blood-brain barrier in cerebral malaria
Authors: TK Darling, PN Mimche, C Bray, B Umaru, LM Brady, C Stone, CE Eboumbou M, TE Lane, LS Ayong, TJ Lamb
PLoS Pathog., 2020-01-30;16(1):e1008261.
Species: Mouse
Sample Types: Whole Cells
Applications: IHC -
Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy
Authors: JH Wu, YN Li, AQ Chen, CD Hong, CL Zhang, HL Wang, YF Zhou, PC Li, Y Wang, L Mao, YP Xia, QW He, HJ Jin, ZY Yue, B Hu
EMBO Mol Med, 2020-01-13;12(2):e10154.
Species: Mouse
Sample Types: Protein
Applications: Western Blot -
A genetic system for tissue-specific inhibition of cell proliferation
Authors: Wenjuan Pu, Ximeng Han, Lingjuan He, Yan Li, Xiuzhen Huang, Mingjun Zhang et al.
Development
-
VEGF/VEGFR2 signaling regulates hippocampal axon branching during development
Authors: Robert Luck, Severino Urban, Andromachi Karakatsani, Eva Harde, Sivakumar Sambandan, LaShae Nicholson et al.
eLife
-
Loss of flow responsive Tie1 results in Impaired Aortic valve remodeling
Authors: Xianghu Qu, Kate Violette, M. K. Sewell-Loftin, Jonathan Soslow, LeShana Saint-Jean, Robert B. Hinton et al.
Developmental Biology
-
Dynamic Interplay between Pericytes and Endothelial Cells during Sprouting Angiogenesis
Authors: G Chiaverina, L di Blasio, V Monica, M Accardo, M Palmiero, B Peracino, M Vara-Messl, A Puliafito, L Primo
Cells, 2019-09-19;8(9):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking
Authors: J Zhang, T Supakornde, JR Krambs, M Rao, G Abou-Ezzi, RY Ye, S Li, K Trinkaus, DC Link
J. Clin. Invest., 2019-04-30;129(7):2920-2931.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Lineage Tracing Reveals the Bipotency of SOX9+ Hepatocytes during Liver Regeneration
Authors: X Han, Y Wang, W Pu, X Huang, L Qiu, Y Li, W Yu, H Zhao, X Liu, L He, L Zhang, Y Ji, J Lu, KO Lui, B Zhou
Stem Cell Reports, 2019-02-14;12(3):624-638.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Erythro-myeloid progenitors contribute endothelial cells to blood vessels
Authors: Alice Plein, Alessandro Fantin, Laura Denti, Jeffrey W. Pollard, Christiana Ruhrberg
Nature
-
Direct Targeting of the mTOR (Mammalian Target of Rapamycin) Kinase Improves Endothelial Permeability in Drug-Eluting Stents—Brief Report
Authors: Emanuel Harari, Liang Guo, Samantha L. Smith, Ka Hyun Paek, Raquel Fernandez, Atsushi Sakamoto et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Role of glutamine synthetase in angiogenesis beyond glutamine synthesis
Authors: G Eelen, C Dubois, AR Cantelmo, J Goveia, U Brüning, M DeRan, G Jarugumill, J van Rijsse, G Saladino, F Comitani, A Zecchin, S Rocha, R Chen, H Huang, S Vandekeere, J Kalucka, C Lange, F Morales-Ro, B Cruys, L Treps, L Ramer, S Vinckier, K Brepoels, S Wyns, J Souffreau, L Schoonjans, WH Lamers, Y Wu, J Haustraete, J Hofkens, S Liekens, R Cubbon, B Ghesquière, M Dewerchin, FL Gervasio, X Li, JD van Buul, X Wu, P Carmeliet
Nature, 2018-08-29;561(7721):63-69.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The bone marrow is patrolled by NK cells that are primed and expand in response to systemic viral activation
Authors: I Milo, R Blecher-Go, Z Barnett-It, R Bar-Ziv, O Tal, I Gurevich, T Feferman, I Drexler, I Amit, P Bousso, G Shakhar
Eur. J. Immunol., 2018-05-02;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Rhodocetin-?? selectively breaks the endothelial barrier of the tumor vasculature in HT1080 fibrosarcoma and A431 epidermoid carcinoma tumor models.
Authors: Stephan Niland, Dorde Komljenov, Jadranka Macas, Thilo Bracht, Tobias Bäuerle, Stefan Liebner, Johannes A Eble
Oncotarget, 2018-04-27;0(0):1949-2553.
Species: Mouse
Sample Types:
Applications: IHC -
Gelatinolytic activity of autocrine matrix metalloproteinase-9 leads to endothelial de-arrangement in Moyamoya disease
Authors: KG Blecharz-L, V Prinz, M Burek, D Frey, T Schenkel, SM Krug, M Fromm, P Vajkoczy
J. Cereb. Blood Flow Metab., 2018-04-10;0(0):271678X187684.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis
Authors: L Guo, H Akahori, E Harari, SL Smith, R Polavarapu, V Karmali, F Otsuka, RL Gannon, RE Braumann, MH Dickinson, A Gupta, AL Jenkins, MJ Lipinski, J Kim, P Chhour, PS de Vries, H Jinnouchi, R Kutys, H Mori, MD Kutyna, S Torii, A Sakamoto, CU Choi, Q Cheng, ML Grove, MA Sawan, Y Zhang, Y Cao, FD Kolodgie, DP Cormode, DE Arking, E Boerwinkle, AC Morrison, J Erdmann, N Sotoodehni, R Virmani, AV Finn
J. Clin. Invest., 2018-02-19;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis
Authors: J Cao, M Ehling, S März, J Seebach, K Tarbashevi, T Sixta, ME Pitulescu, AC Werner, B Flach, E Montanez, E Raz, RH Adams, H Schnittler
Nat Commun, 2017-12-20;8(1):2210.
Species: Mouse, Rat
Sample Types: Whole Tissue
Applications: IHC -
Embryonic Stem Cell Differentiation to Functional Arterial Endothelial Cells through Sequential Activation of ETV2 and NOTCH1 Signaling by HIF1?
Authors: KM Tsang, JS Hyun, KT Cheng, M Vargas, D Mehta, M Ushio-Fuka, L Zou, KV Pajcini, J Rehman, AB Malik
Stem Cell Reports, 2017-08-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Niche-mediated depletion of the normal hematopoietic stem cell reservoir by Flt3-ITD-induced myeloproliferation
Authors: AJ Mead, WH Neo, N Barkas, S Matsuoka, A Giustacchi, R Facchini, S Thongjuea, L Jamieson, CAG Booth, N Fordham, C Di Genua, D Atkinson, O Chowdhury, E Repapi, N Gray, S Kharazi, SA Clark, T Bouriez, P Woll, T Suda, C Nerlov, SEW Jacobsen
J. Exp. Med., 2017-06-21;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming
Authors: L Tian, A Goldstein, H Wang, H Ching Lo, I Sun Kim, T Welte, K Sheng, LE Dobrolecki, X Zhang, N Putluri, TL Phung, SA Mani, F Stossi, A Sreekumar, MA Mancini, WK Decker, C Zong, MT Lewis, XH Zhang
Nature, 2017-04-03;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
HDL activation of endothelial sphingosine-1-phosphate receptor-1 (S1P1) promotes regeneration and suppresses fibrosis in the liver
JCI Insight, 2016-12-22;1(21):e87058.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
PAR1 Scaffolds TGF beta RII to Downregulate TGF-beta Signaling and Activate ESC Differentiation to Endothelial Cells
Authors: Haixia Gong, Shejuan An, Antonia Sassmann, Menglin Liu, Victoria Mastej, Manish Mittal et al.
Stem Cell Reports
-
Endothelial cells are progenitors of cardiac pericytes and vascular smooth muscle cells
Nat Commun, 2016-08-12;7(0):12422.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Targeting of Mesenchymal Stromal Cells by Cre-Recombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells
Authors: Jingzhu Zhang
J Bone Miner Res, 2016-06-14;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes.
Authors: Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Zhang L, Qiao Z, Wang Q, Lui K, Zhou B
Cell Res, 2015-12-04;26(1):119-30.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Granzyme B Deficiency Protects against Angiotensin II-Induced Cardiac Fibrosis.
Authors: Shen Y, Cheng F, Sharma M, Merkulova Y, Raithatha S, Parkinson L, Zhao H, Westendorf K, Bohunek L, Bozin T, Hsu I, Ang L, Williams S, Bleackley R, Eriksson J, Seidman M, McManus B, Granville D
Am J Pathol, 2015-11-25;186(1):87-100.
Species: Mouse
Sample Types: Recombinant Protein, Whole Tissue
Applications: IHC-P, Western Blot -
Fibroblast Growth Factor 9 Imparts Hierarchy and Vasoreactivity to the Microcirculation of Renal Tumors and Suppresses Metastases.
Authors: Yin H, Frontini M, Arpino J, Nong Z, O'Neil C, Xu Y, Balint B, Ward A, Chakrabarti S, Ellis C, Gros R, Pickering J
J Biol Chem, 2015-07-16;290(36):22127-42.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Vascular dysfunction and increased metastasis of B16F10 melanomas in Shb deficient mice as compared with their wild type counterparts.
Authors: Zang G, Gustafsson K, Jamalpour M, Hong J, Genove G, Welsh M
BMC Cancer, 2015-04-08;15(0):234.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration
Authors: Shahin Rafii, Zhongwei Cao, Raphael Lis, Ilias I. Siempos, Deebly Chavez, Koji Shido et al.
Nature Cell Biology
-
Neuroligin 1 induces blood vessel maturation by cooperating with the alpha6 integrin.
Authors: Samarelli A, Riccitelli E, Bizzozero L, Silveira T, Seano G, Pergolizzi M, Vitagliano G, Cascone I, Carpentier G, Bottos A, Primo L, Bussolino F, Arese M
J Biol Chem, 2014-05-23;289(28):19466-76.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Blood vessels pattern heparan sulfate gradients between their apical and basolateral aspects.
Authors: Stoler-Barak, Liat, Moussion, Christin, Shezen, Elias, Hatzav, Miki, Sixt, Michael, Alon, Ronen
PLoS ONE, 2014-01-22;9(1):e85699.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Flk1+ and VE-cadherin+ endothelial cells derived from iPSCs recapitulates vascular development during differentiation and display similar angiogenic potential as ESC-derived cells.
Authors: Kohler, Erin E, Wary, Kishore, Li, Fei, Chatterjee, Ishita, Urao, Norifumi, Toth, Peter T, Ushio-Fukai, Masuko, Rehman, Jalees, Park, Changwon, Malik, Asrar B
PLoS ONE, 2013-12-30;8(12):e85549.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry, ICC -
Transcriptional targeting of primary and metastatic tumor neovasculature by an adenoviral type 5 roundabout4 vector in mice.
Authors: Lu, Zhi Hong, Kaliberov, Sergey, Sohn, Rebecca, Kaliberova, Lyudmila, Curiel, David T, Arbeit, Jeffrey
PLoS ONE, 2013-12-23;8(12):e83933.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: Western Blot -
Control of retinoid levels by CYP26B1 is important for lymphatic vascular development in the mouse embryo.
Authors: Bowles J, Secker G, Nguyen C, Kazenwadel J, Truong V, Frampton E, Curtis C, Skoczylas R, Davidson T, Miura N, Hong Y, Koopman P, Harvey N, Francois M
Dev Biol, 2013-12-19;386(1):25-33.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis.
Authors: Ding B, Cao Z, Lis R, Nolan D, Guo P, Simons M, Penfold M, Shido K, Rabbany S, Rafii S
Nature, 2013-11-20;505(7481):97-102.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd.
Authors: Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, Sundvold-Gjerstad V, Gustafsson K, Choy WW, Zang G, Quach M, Jansson L, Phillipson M, Abid MR, Spurkland A, Claesson-Welsh L
J. Exp. Med., 2012-06-11;209(7):1363-77.
Species: Mouse
Sample Types: Tissue Homogenates, Whole Tissue
Applications: IHC, Western Blot -
Unique Cell Type-Specific Junctional Complexes in Vascular Endothelium of Human and Rat Liver Sinusoids
Authors: Cyrill Géraud, Konstantin Evdokimov, Beate K. Straub, Wiebke K. Peitsch, Alexandra Demory, Yvette Dörflinger et al.
PLoS ONE
-
Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice.
Authors: Marrotte EJ, Chen DD, Hakim JS
J. Clin. Invest., 2010-11-08;120(12):4207-19.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells.
Authors: Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S
Cell Stem Cell, 2010-03-05;6(3):251-64.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr, IHC-P -
Dysfunctional microvasculature as a consequence of shb gene inactivation causes impaired tumor growth.
Authors: Funa NS, Kriz V, Zang G, Calounova G, Akerblom B, Mares J, Larsson E, Sun Y, Betsholtz C, Welsh M
Cancer Res., 2009-02-17;69(5):2141-8.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization.
Authors: Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P
Cell, 2009-02-12;136(5):839-51.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays.
Authors: Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP
Stem Cells, 2008-07-31;26(10):2586-94.
Species: Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC-Fr -
Genetic delivery of the murine equivalent of bevacizumab (avastin), an anti-vascular endothelial growth factor monoclonal antibody, to suppress growth of human tumors in immunodeficient mice.
Authors: Watanabe M, Boyer JL, Hackett NR, Qiu J, Crystal RG
Hum. Gene Ther., 2008-03-01;19(3):300-10.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Lentiviral rescue of vascular endothelial growth factor receptor-2 expression in flk1-/- embryonic stem cells shows early priming of endothelial precursors.
Authors: Li X, Edholm D, Lanner F, Breier G, Farnebo F, Dimberg A, Claesson-Welsh L
Stem Cells, 2007-08-16;25(12):2987-95.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Effect of gestational nutrition on vascular integrity in the murine placenta.
Authors: Rutland CS, Latunde-Dada AO, Thorpe A, Plant R, Langley-Evans S, Leach L
Placenta, 2006-08-23;28(7):734-42.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy
Authors: J Hu, S Dziumbla, J Lin, SI Bibli, S Zukunft, J de Mos, K Awwad, T Frömel, A Jungmann, K Devraj, Z Cheng, L Wang, S Fauser, CG Eberhart, A Sodhi, BD Hammock, S Liebner, OJ Müller, C Glaubitz, HP Hammes, R Popp, I Fleming
Nature, 2017-12-06;552(7684):248-252.
-
Semaphorin 7A Promotes VEGFA/VEGFR2-Mediated Angiogenesis and Intraplaque Neovascularization in ApoE-/- Mice
Authors: Shuhong Hu, Yifei Liu, Tao You, Li Zhu
Frontiers in Physiology
-
Regeneration of the pulmonary vascular endothelium after viral pneumonia requires COUP-TF2
Authors: Gan Zhao, Aaron I. Weiner, Katherine M. Neupauer, Maria Fernanda de Mello Costa, Gargi Palashikar, Stephanie Adams-Tzivelekidis et al.
Science Advances
-
Rasip1 regulates vertebrate vascular endothelial junction stability through Epac1-Rap1 signaling.
Authors: Wilson CW, Parker LH, Hall CJ et al.
Blood.
-
Vascular Permeability Assay in Human Coronary and Mouse Brachiocephalic Arteries.
Authors: Guo L, Fernandez R, Sakamoto A et al.
Stem Cells International.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse VE-Cadherin Antibody
Average Rating: 4 (Based on 1 Review)
Have you used Mouse VE-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: