Mouse Fc gamma RI/CD64 Antibody Summary
Glu25-Pro297
Accession # P26151
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
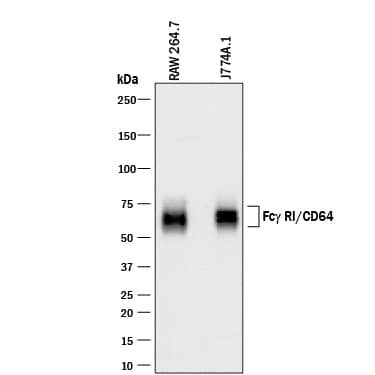 View Larger
View Larger
Detection of Mouse Fc gamma RI/CD64 by Western Blot. Western blot shows lysates of RAW 264.7 mouse monocyte/macrophage cell line and J774A.1 mouse reticulum cell sarcoma macrophage cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Mouse Fc gamma RI/CD64 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2074) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for Fc gamma RI/CD64 at approximately 60-75 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
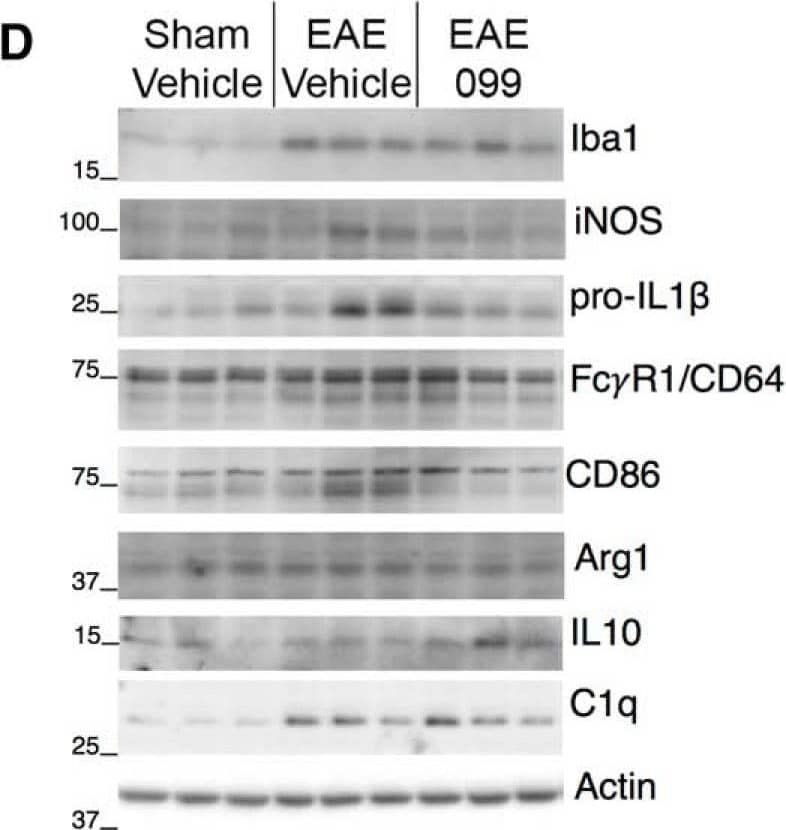 View Larger
View Larger
Detection of Mouse Fc gamma RI/CD64 by Western Blot URMC-099 modulates the phenotype of activated microglia in EAE hippocampus. A, Staining for Iba1 in hippocampal area CA1 shows ramified microglia in sham-immunized mice, and microglia with shorter, thicker processes in EAE. B, Iba1-positive cells in the hippocampi of both sham and EAE mice coexpress microglia-specific marker Tmem119 along their processes and to a more variable extent in the cell body (upper panels). Iba1-positive, Tmem119-negative cells consistent with monocyte-derived macrophages could be found in the pia during EAE (inset), but not in or around the hippocampus. Staining for Ly-6B, expressed on neutrophils and some recently-generated macrophages, is sparse in sham and EAE hippocampi (lower panels, arrowheads), although clusters occurred sporadically in the pia and other area of some EAE brains (inset). Scale bars: 20 µm. s.o., CA1 stratum oriens; pyr, pyramidal layer; s.r., stratum radiatum. C, Quantification of microglial images shows increased Iba1 intensity and reduction in the ratio of surface area to volume in EAE microglia regardless of treatment with URMC-099 or CLFB1134. Microglial expression of lysosomal marker CD68 is increased in many hippocampi from vehicle-treated EAE mice but not in mice treated with URMC-099 or CLFB1134; n = 10 male mice (sham-vehicle), n = 6 male mice (EAE-vehicle and EAE-099), n = 7 male mice (EAE-1134). D, E, Western blottings and band densitometry for markers of inflammation and microglial phenotype in hippocampal protein extracts. Markers associated with proinflammatory microglial activation tended to increase in vehicle-treated EAE hippocampi and were decreased by URMC-099, with significant changes in iNOS (inducible nitric oxide synthase) and immunoglobulin receptor FC gamma R1/CD64 (lower single band), and a non-significant trend toward increased pro-IL1 beta. Proinflammatory marker CD86 does not significantly increase in vehicle-treated EAE but is decreased by URMC-099 treatment. Arg1 and IL-10, canonical markers of anti-inflammatory alternative activation, are not significantly changed in EAE or with URMC-099. Consistent with immunostaining results, Iba1 is up-regulated in vehicle-treated EAE hippocampi and is further increased with URMC-099 treatment. Complement component C1q follows a similar pattern; n = 10 male mice (sham-vehicle), n = 7 male mice (EAE-vehicle), n = 11 male mice (EAE-099); one representative blot with three lanes per condition is depicted; *p < 0.05, **p < 0.01, one-way ANOVA with Sidak (iNOS, FC gamma R1/CD64, CD86) or Tukey (Iba1, SA/Vol, C1q) post hoc test. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30627663), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: Fc gamma RI/CD64
Receptors for the Fc region of IgG (Fc gamma Rs) are members of the Ig superfamily that function in the activation or inhibition of immune responses such as degranulation, phagocytosis, ADCC (antibody-dependent cellular toxicity), cytokine release, and B cell proliferation (1‑3). The Fc gamma Rs have been divided into three classes based on close relationships in their extracellular domains; these groups are designated Fc gamma RI (also known as CD64), Fc gamma RII (CD32), and Fc gamma RIII (CD16). Each group may be encoded by multiple genes and exist in different isoforms depending on species and cell type. The CD64 proteins are high affinity receptors (~10‑8‑10‑9 M) capable of binding monomeric IgG, whereas the CD16 and CD32 proteins bind IgG with lower affinities (~10-6‑10-7 M) only recognizing IgG aggregates surrounding multivalent antigens (1, 4). Fc gamma Rs that deliver an activating signal either have an intrinsic immunoreceptor tyrosine‑based activation motif (ITAM) within their cytoplasmic domains or associate with one of the ITAM-bearing adapter subunits, Fc R gamma or zeta (3, 5). The only inhibitory member in human and mouse, Fc gamma RIIb, has an intrinsic cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM). The coordinated functioning of activating and inhibitory receptors is necessary for successful initiation, amplification, and termination of immune responses (5).
Mouse Fc gamma RI is transmembrane protein with three extracellular Ig-like domains, and it delivers an activating signal via the associated Fc R gamma accessory chain (1, 2). The high affinity recognition of IgG by Fc gamma RI permits the triggering of effector responses at low IgG concentrations typical of early immune responses (2). Fc gamma RI is expressed constitutively on monocytes and macrophages and can be induced on neutrophils and eosinophils (1, 4). Its expression is up-regulated during bacterial infections and sepsis.
- Van de Winkel, J. and P. Capes (1993) Immunol. Today 14:215.
- Raghaven, M. and P. Bjorkman (1996) Annu. Rev. Cell Dev. Biol. 12:181.
- Ravetch, J. and S. Bolland (2001) Annu. Rev. Immunol. 19:275.
- Takai, T. (2002) Nature Rev. Immunol. 2:580.
- Ravetch, J. and L. Lanier (2000) Science 290:84.
Product Datasheets
Citations for Mouse Fc gamma RI/CD64 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
7
Citations: Showing 1 - 7
Filter your results:
Filter by:
-
Cartilage-binding antibodies induce pain through immune complex–mediated activation of neurons
Authors: Alex Bersellini Farinotti, Gustaf Wigerblad, Diana Nascimento, Duygu B. Bas, Carlos Morado Urbina, Kutty Selva Nandakumar et al.
Journal of Experimental Medicine
-
Fc gamma receptor activation mediates vascular inflammation and abdominal aortic aneurysm development
Authors: Laura Lopez‐Sanz, Susana Bernal, Luna Jimenez‐Castilla, Ignacio Prieto, Sara La Manna, Sergio Gomez‐Lopez et al.
Clinical and Translational Medicine
-
Surface Proteins of SARS-CoV-2 Drive Airway Epithelial Cells to Induce IFN-Dependent Inflammation
Authors: Gautam Anand, Alexandra M. Perry, Celeste L. Cummings, Emma St. Raymond, Regina A. Clemens, Ashley L. Steed
The Journal of Immunology
-
FFAR4 regulates cardiac oxylipin balance to promote inflammation resolution in HFpEF secondary to metabolic syndrome
Authors: Naixin Zhang, Brian Harsch, Michael J. Zhang, Dylan J. Gyberg, Jackie A. Stevens, Brandon M. Wagner et al.
Journal of Lipid Research
-
Optimization of Whole Tumor Cell Vaccines by Interaction with Phagocytic Receptors
Authors: M Korbelik
Vaccines, 2021-08-14;9(8):.
Species: Mouse
Sample Types: In Vivo
Applications: Neutralization -
Intravenous nanoparticle vaccination generates stem-like TCF1+ neoantigen-specific CD8+ T cells
Authors: F Baharom, RA Ramirez-Va, KKS Tobin, H Yamane, CA Dutertre, A Khalilnezh, GV Reynoso, VL Coble, GM Lynn, MP Mulè, AJ Martins, JP Finnigan, XM Zhang, JA Hamerman, N Bhardwaj, JS Tsang, HD Hickman, F Ginhoux, AS Ishizuka, RA Seder
Nat Immunol, 2020-11-02;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The Mixed-Lineage Kinase Inhibitor URMC-099 Protects Hippocampal Synapses in Experimental Autoimmune Encephalomyelitis
Authors: Matthew J. Bellizzi, Jennetta W. Hammond, Herman Li, Mary A. Gantz Marker, Daniel F. Marker, Robert S. Freeman et al.
eNeuro
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse Fc gamma RI/CD64 Antibody
There are currently no reviews for this product. Be the first to review Mouse Fc gamma RI/CD64 Antibody and earn rewards!
Have you used Mouse Fc gamma RI/CD64 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
