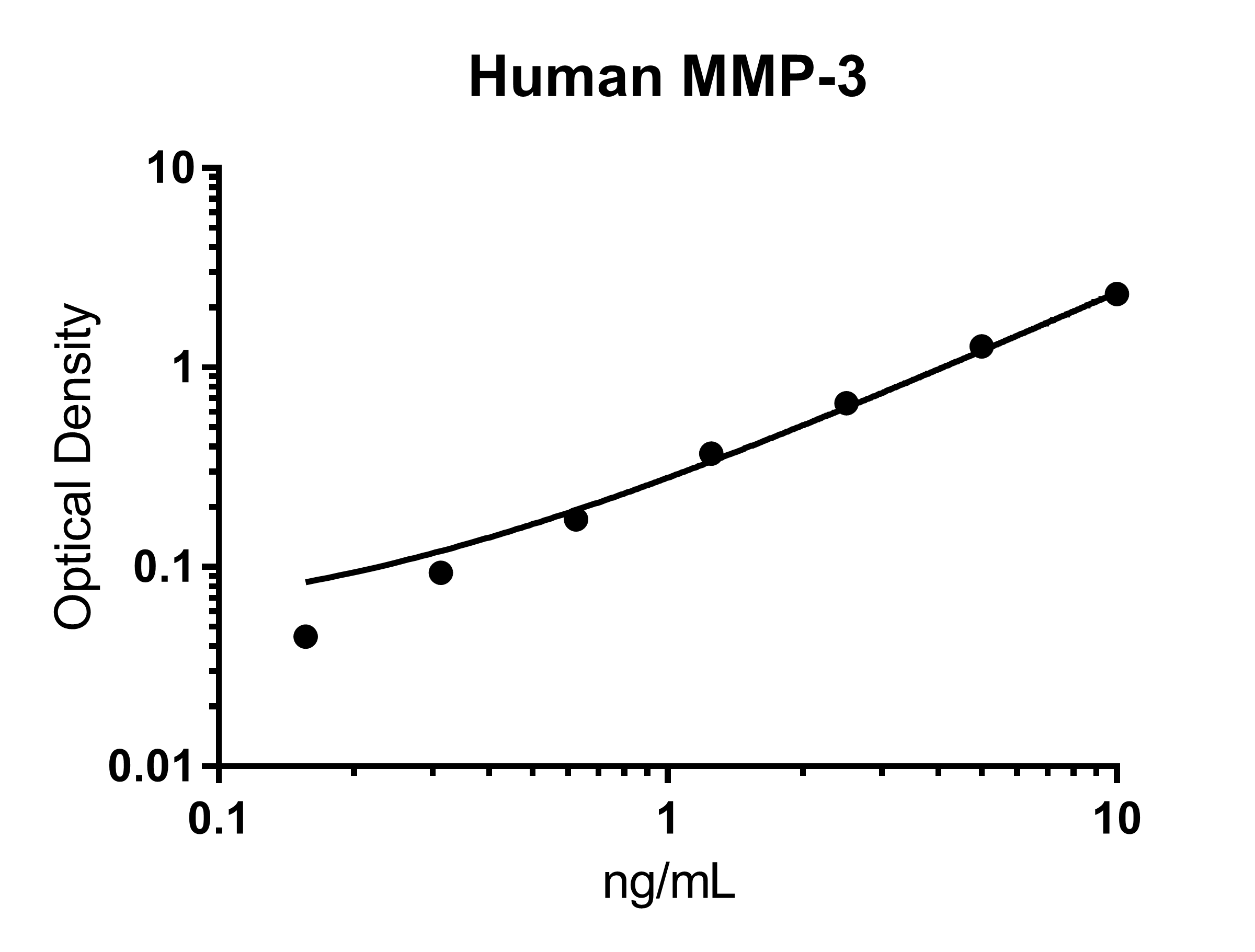
Human Total MMP-3 Quantikine ELISA Kit Summary
Product Summary
Recovery
The recovery of MMP-3 spiked to levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=4) | 96 | 87-104 |
| Heparin Plasma (n=5) | 92 | 85-106 |
| Serum (n=5) | 90 | 86-96 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: MMP-3
Matrix metalloproteinases (MMPs), also called matrixins, constitute a family of zinc and calcium dependent endopeptidases that function in the breakdown of extracellular matrix (ECM). They play an important role in many normal physiological processes such as embryonic development, morphogenesis, reproduction and tissue remodeling (1). They also participate in many pathological processes such as arthritis, cancer and cardiovascular disease (2). While the amounts of newly synthesized MMPs are regulated mainly at the levels of transcription, the proteolytic activities of existing MMPs are controlled through both the activation of proenzymes or zymogens and the inhibition of active enzymes by endogenous inhibitors, alpha -macroglobulins and tissue inhibitors of metalloproteinases (TIMPs).
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours on a horizontal orbital microplate shaker.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours on the shaker.
- Aspirate and wash 4 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes on the benchtop. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human Total MMP-3 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
53
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Umbilical Cord Mesenchymal Stem Cell Secretome Improves Clinical Outcomes and Changes Biomarkers in Knee Osteoarthritis
Authors: Partan, RU;Putra, KM;Kusuma, NF;Darma, S;Reagan, M;Muthia, P;Radiandina, AS;Saleh, MI;Salim, EM;
Journal of clinical medicine
Species: Human
Sample Types: Whole Blood
-
Matrix metalloproteinase-3 (MMP-3)-mediated gene therapy for glaucoma
Authors: J O'Callagha, C Delaney, M O'Connor, J van Batenb, M Schicht, E Lütjen-Dre, N Hudson, S Ni Dhubhgh, P Humphries, C Stanley, A Keravala, T Chalberg, MS Lawrence, M Campbell
Science Advances, 2023-04-19;9(16):eadf6537.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix Metalloproteinases Contribute to the Calcification Phenotype in Pseudoxanthoma Elasticum
Authors: Pl�mers, R;Lindenkamp, C;Osterhage, MR;Knabbe, C;Hendig, D;
Biomolecules
Species: Human
Sample Types: Cell Culture Supernates
-
Microfluidic Electroceuticals Platform for Therapeutic Strategies of Intervertebral Disc Degeneration: Effects of Electrical Stimulation on Human Nucleus Pulposus Cells under Inflammatory Conditions
Authors: TW Kim, AG Kim, KH Lee, MH Hwang, H Choi
International Journal of Molecular Sciences, 2022-09-04;23(17):.
Species: Human
Sample Types: Cell Culture Supernates
-
Adipokine human Resistin promotes obesity-associated inflammatory intervertebral disc degeneration via pro-inflammatory cytokine cascade activation
Authors: JH Shin, S Park, H Cho, JH Kim, H Choi
Scientific Reports, 2022-05-27;12(1):8936.
Species: Human
Sample Types: Cell Culture Supernates
-
Relationship between different serum cartilage biomarkers in the acute response to running and jumping in healthy male individuals
Authors: M Dreiner, T Munk, F Zaucke, AM Liphardt, A Niehoff
Scientific Reports, 2022-04-19;12(1):6434.
Species: Human
Sample Types: Serum
-
Early IL-17A production helps establish Mycobacterium intracellulare infection in mice
Authors: BG Jung, B Samten, K Dean, RJ Wallace, BA Brown-Elli, T Tucker, S Idell, JV Philley, R Vankayalap
PloS Pathogens, 2022-04-01;18(4):e1010454.
Species: Human
Sample Types: Tissue Homogenates
-
Concentration of Selected Metalloproteinases and Osteocalcin in the Serum and Synovial Fluid of Obese Women with Advanced Knee Osteoarthritis
Authors: J Jarecki, T Ma?ecka-Ma, E Kosior-Jar, W Widuchowsk, P Krasowski, M Gutbier, M Dobrzy?ski, T Blicharski
International Journal of Environmental Research and Public Health, 2022-03-16;19(6):.
Species: Human
Sample Types: Serum
-
Tumor necrosis factor alpha neutralization attenuates immune checkpoint inhibitor-induced activation of intermediate monocytes in synovial fluid mononuclear cells from patients with inflammatory arthritis
Authors: AS Sørensen, MN Andersen, K Juul-Madse, AD Broksø, C Skejø, H Schmidt, T Vorup-Jens, TW Kragstrup
Arthritis Research & Therapy, 2022-02-14;24(1):43.
Species: Human
Sample Types: Cell Culture Supernates
-
Baseline serum biomarkers of inflammation, bone turnover and adipokines predict spinal radiographic progression in ankylosing spondylitis patients on TNF inhibitor therapy
Authors: J Rademacher, M Siderius, L Gellert, FR Wink, M Verba, F Maas, LM Tietz, D Poddubnyy, A Spoorenber, S Arends
Seminars in arthritis and rheumatism, 2022-02-02;53(0):151974.
Species: Human
Sample Types: Serum
-
Enhanced Expression but Decreased Specific Activity of Matrix Metalloproteinase 10 (MMP-10) in Comparison with Matrix Metalloproteinase 3 (MMP-3) in Human Urinary Bladder Carcinoma
Authors: J Kudelski, G M?ynarczyk, M Gudowska-S, B Mroczko, B Darewicz, M Bruczko-Go, K Sobolewski, L Romanowicz
Journal of Clinical Medicine, 2021-08-19;10(16):.
Species: Human
Sample Types: Tissue Homogenates
-
The Migration of Human Follicular Dendritic Cell-Like Cell Is Facilitated by Matrix Metalloproteinase 3 Expression That Is Mediated through TNFalpha-ERK1/2-AP1 Signaling
Authors: HK Pak, YW Kim, B Nam, AN Lee, J Roh, M Gil, C Liu, YS Chung, CS Park
Journal of Immunology Research, 2021-06-17;2021(0):8483938.
Species: Human
Sample Types: Cell Culture Supernates
-
Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis
Authors: K Chihara, N Hattori, N Ichikawa, T Matsuda, T Saito
Sci Rep, 2020-09-28;10(1):15932.
Species: Human
Sample Types: Serum
-
Activity of fibroblast-like synoviocytes in rheumatoid arthritis was impaired by dickkopf-1 targeting siRNA
Authors: YY Liu, SY Wang, YN Li, WJ Bian, LQ Zhang, YH Li, L Long, X Liu, XW Zhang, ZG Li
Chin. Med. J., 2020-03-20;0(6):679-686.
Species: Human
Sample Types: Cell Culture Supernates
-
Basilar Artery Tortuosity Is Associated With White Matter Hyperintensities by TIMP-1
Authors: DP Zhang, YF Peng, HL Zhang, JG Ma, M Zhao, S Yin, TT Wei
Front Neurosci, 2019-08-14;13(0):836.
Species: Human
Sample Types: Plasma
-
Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging
Authors: DP Muñoz, SM Yannone, A Daemen, Y Sun, F Vakar-Lope, M Kawahara, AM Freund, F Rodier, JD Wu, PY Desprez, DH Raulet, PS Nelson, LJ van 't Vee, J Campisi, JP Coppé
JCI Insight, 2019-06-11;5(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Increased levels of circulating MMP3 correlate with severe rejection in face transplantation
Authors: B Kollar, A Shubin, TJ Borges, S Tasigiorgo, TS Win, CG Lian, ST Dillon, X Gu, I Wyrobnik, GF Murphy, B Pomahac, TA Libermann, LV Riella
Sci Rep, 2018-10-08;8(1):14915.
Species: Human
Sample Types: Serum
-
Two independent proteomic approaches provide a comprehensive analysis of the synovial fluid proteome response to Autologous Chondrocyte Implantation
Authors: CH Hulme, EL Wilson, HR Fuller, S Roberts, JB Richardson, P Gallacher, MJ Peffers, SL Shirran, CH Botting, KT Wright
Arthritis Res. Ther., 2018-05-02;20(1):87.
Species: Human
Sample Types: Synovial Fluid
-
PhosphoLipid transfer protein (PLTP) exerts a direct pro-inflammatory effect on rheumatoid arthritis (RA) fibroblasts-like-synoviocytes (FLS) independently of its lipid transfer activity
Authors: R Audo, V Deckert, CI Daien, H Che, J Elhmioui, S Lemaire, JP Pais de Ba, C Desrumaux, B Combe, M Hahne, L Lagrost, J Morel
PLoS ONE, 2018-03-22;13(3):e0193815.
Species: Human
Sample Types: Cell Culture Supernates
-
Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis
Authors: C Robinson, L Tsang, A Solomon, AJ Woodiwiss, S Gunter, M Mer, HC Hsu, M Gomes, GR Norton, AME Millen, PH Dessein
Peptides, 2018-02-21;102(0):31-37.
Species: Human
Sample Types: Plasma
-
CX3CL1 promotes MMP-3 production via the CX3CR1, c-Raf, MEK, ERK, and NF-?B signaling pathway in osteoarthritis synovial fibroblasts
Authors: SM Hou, CH Hou, JF Liu
Arthritis Res. Ther., 2017-12-21;19(1):282.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum bone-turnover biomarkers are associated with the occurrence of peripheral and axial arthritis in psoriatic disease: a prospective cross-sectional comparative study
Authors: DR Jadon, R Sengupta, A Nightingal, H Lu, J Dunphy, A Green, JT Elder, RP Nair, E Korendowyc, MA Lindsay, NJ McHugh
Arthritis Res. Ther., 2017-09-21;19(1):210.
Species: Human
Sample Types: Serum
-
Late mitral restenosis after percutaneous commissurotomy: Predictive value of inflammation and extracellular matrix remodeling biomarkers
Authors: R Mechmeche, A Zaroui, S Aloui, M Boukhris, M Allal-Elas, N Kaabachi, B Zouari
Heart Lung, 2017-04-24;0(0):.
Species: Human
Sample Types: Serum
-
Concomitant elevations of MMP-9, NGAL, proMMP-9/NGAL and neutrophil elastase in serum of smokers with chronic obstructive pulmonary disease
J. Cell. Mol. Med, 2016-12-22;0(0):.
Species: Human
Sample Types: Serum
-
Galectin-3 Induces a Pro-degradative/inflammatory Gene Signature in Human Chondrocytes, Teaming Up with Galectin-1 in Osteoarthritis Pathogenesis
Sci Rep, 2016-12-16;6(0):39112.
Species: Human
Sample Types: Cell Culture Supernates
-
Exosomes in Human Breast Milk Promote EMT
Authors: W Qin, Y Tsukasaki, S Dasgupta, N Mukhopadhy, M Ikebe, ER Sauter
Clin Cancer Res, 2016-04-08;0(0):.
Species: Human
Sample Types: Cell Lysates
-
Metallothionein-3 Increases Triple-Negative Breast Cancer Cell Invasiveness via Induction of Metalloproteinase Expression.
Authors: Kmiecik A, Pula B, Suchanski J, Olbromski M, Gomulkiewicz A, Owczarek T, Kruczak A, Ambicka A, Rys J, Ugorski M, Podhorska-Okolow M, Dziegiel P
PLoS ONE, 2015-05-01;10(5):e0124865.
Species: Human
Sample Types: Cell Culture Supernates
-
Exogenous IFN-beta regulates the RANKL-c-Fos-IFN-beta signaling pathway in the collagen antibody-induced arthritis model.
Authors: Zhao R, Chen N, Zhou X, Miao P, Hu C, Qian L, Yu Q, Zhang J, Nie H, Chen X, Li P, Xu R, Xiao L, Zhang X, Liu J, Zhang D
J Transl Med, 2014-12-10;12(1):330.
Species: Human
Sample Types: Serum
-
Interleukin-6 and matrix metalloproteinase expression in the subretinal fluid during proliferative vitreoretinopathy: correlation with extent, duration of RRD and PVR grade.
Authors: Symeonidis C, Papakonstantinou E, Androudi S, Tsaousis KT, Tsinopoulos I, Brazitikos P, Karakiulakis G, Diza E, Dimitrakos SA
Cytokine, 2012-05-10;59(1):184-90.
Species: Human
Sample Types: Subretinal Fluid
-
Validation and quality control of ELISAs for the use with human saliva samples.
Authors: Jaedicke KM, Taylor JJ, Preshaw PM
J. Immunol. Methods, 2012-01-28;377(1):62-5.
Species: Human
Sample Types: Saliva
-
Interleukin-6 and the matrix metalloproteinase response in the vitreous during proliferative vitreoretinopathy.
Authors: Symeonidis C, Papakonstantinou E, Androudi S, Rotsos T, Diza E, Brazitikos P, Karakiulakis G, Dimitrakos SA
Cytokine, 2011-02-25;54(2):212-7.
Species: Human
Sample Types: Vitreous Humor
-
Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells.
Authors: Yamakawa N, Uchida T, Matthay MA, Makita K
Am. J. Physiol. Lung Cell Mol. Physiol., 2011-01-21;300(4):L516-25.
Species: Human
Sample Types: Edema Fluid
-
Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes.
Authors: Verma S, Kumar M, Nerurkar VR
J. Gen. Virol., 2010-11-24;92(0):507-15.
Species: Human
Sample Types: Cell Culture Supernates
-
High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1.
Authors: Hashizume M, Mihara M
Biochem. Biophys. Res. Commun., 2010-11-06;403(2):184-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Matrix metalloproteinase-9 and plasminogen activator inhibitor-1 are associated with right ventricular structure and function: the MESA-RV Study.
Authors: Kawut SM, Barr RG, Johnson WC
Biomarkers, 2010-10-05;15(8):731-8.
Species: Human
Sample Types: Plasma
-
Soluble biomarkers of cartilage and bone metabolism in early proof of concept trials in psoriatic arthritis: effects of adalimumab versus placebo.
Authors: van Kuijk AW, DeGroot J, Koeman RC
PLoS ONE, 2010-09-03;5(9):e12556.
Species: Human
Sample Types: Serum
-
Circulating matrix metalloproteinase-3 and metalloproteinase-9 and tissue Doppler measures of diastolic dysfunction to risk stratify patients with systolic heart failure.
Authors: Buralli S, Dini FL, Ballo P, Conti U, Fontanive P, Duranti E, Metelli MR, Marzilli M, Taddei S
Am. J. Cardiol., 2010-03-15;105(6):853-6.
Species: Human
Sample Types: Plasma
-
Primary human acute myelogenous leukemia cells release matrix metalloproteases and their inhibitors: release profile and pharmacological modulation.
Authors: Reikvam H, Hatfield KJ, Oyan AM, Kalland KH, Kittang AO, Bruserud O
Eur. J. Haematol., 2009-11-17;84(3):239-51.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of matrix metalloproteinases -1,-2,-3 and -9 in thoracic aortic diseases and acute myocardial ischemia.
Authors: Karapanagiotidis GT, Antonitsis P, Charokopos N, Foroulis CN, Anastasiadis K, Rouska E, Argiriadou H, Rammos K, Papakonstantinou C
J Cardiothorac Surg, 2009-11-03;4(0):59.
Species: Human
Sample Types: Serum
-
PI3Kgamma regulates cartilage damage in chronic inflammatory arthritis.
Authors: Hayer S, Pundt N, Peters MA, Wunrau C, Kuhnel I, Neugebauer K, Strietholt S, Zwerina J, Korb A, Penninger J, Joosten LA, Gay S, Ruckle T, Schett G, Pap T
FASEB J., 2009-09-04;23(12):4288-98.
Species: Human
Sample Types: Cell Culture Supernates
-
Establishment of a matrix-associated transepithelial resistance invasion assay to precisely measure the invasive potential of synovial fibroblasts.
Authors: Wunrau C, Schnaeker EM, Freyth K, Pundt N, Wendholt D, Neugebauer K, Hansen U, Pap T, Dankbar B
Arthritis Rheum., 2009-09-01;60(9):2606-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated serum matrix metalloproteinase-3 and -7 in H. pylori-related gastric cancer can be biomarkers correlating with a poor survival.
Authors: Yeh YC, Sheu BS, Cheng HC, Wang YL, Yang HB, Wu JJ
Dig. Dis. Sci., 2009-08-19;55(6):1649-57.
Species: Human
Sample Types: Serum
-
Inhibition of IL-1beta-mediated inflammatory responses by the IkappaB alpha super-repressor in human fibroblast-like synoviocytes.
Authors: Lee YR, Kweon SH, Kwon KB, Park JW, Yoon TR, Park BH
Biochem. Biophys. Res. Commun., 2008-11-11;378(1):90-4.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of MMP-3 and cathepsin K in patients with ankylosing spondylitis: effect of TNFalpha antagonist therapy.
Authors: Wendling D, Cedoz JP, Racadot E
Joint Bone Spine, 2008-10-01;75(5):559-62.
Species: Human
Sample Types: Serum
-
Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer.
Authors: Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G, Sharifi S, Kuliopulos A
Mol. Cancer Ther., 2008-09-01;7(9):2746-57.
Species: Human
Sample Types: Ovarian Ascites Fluid
-
Gender specific associations between matrix metalloproteinases and inflammatory markers in post myocardial infarction patients.
Authors: Samnegard A, Hulthe J, Silveira A, Ericsson CG, Hamsten A, Eriksson P
Atherosclerosis, 2008-06-05;202(2):550-6.
Species: Human
Sample Types: Serum
-
Actinobacillus actinomycetemcomitans lipopolysaccharide regulates matrix metalloproteinase, tissue inhibitors of matrix metalloproteinase, and plasminogen activator production by human gingival fibroblasts: a potential role in connective tissue destruction.
Authors: Bodet C, Andrian E, Tanabe S, Grenier D
J. Cell. Physiol., 2007-07-01;212(1):189-94.
Species: Human
Sample Types: Cell Culture Supernates
-
Advanced glycation end products decrease mesangial cell MMP-7: a role in matrix accumulation in diabetic nephropathy?
Authors: McLennan SV, Kelly DJ, Schache M, Waltham M, Dy V, Langham RG, Yue DK, Gilbert RE
Kidney Int., 2007-06-06;72(4):481-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro.
Authors: Zhao Z, McCloud B, Fleming R, Klempner MS
Biochem. Biophys. Res. Commun., 2007-05-02;358(2):528-33.
Species: Human
Sample Types: Tissue Homogenates
-
Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-alpha in human osteoarthritic chondrocytes.
Authors: Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK
FEBS Lett., 2007-04-09;581(9):1928-32.
Species: Human
Sample Types: Cell Culture Supernates
-
Gingival fibroblasts inhibit MMP-1 and MMP-3 activities in an ex-vivo artery model.
Authors: Naveau A, Reinald N, Fournier B
Connect. Tissue Res., 2007-01-01;48(6):300-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells.
Authors: Li DQ, Chen Z, Song XJ, Luo L, Pflugfelder SC
Invest. Ophthalmol. Vis. Sci., 2004-12-01;45(12):4302-11.
Species: Human
Sample Types: Cell Culture Supernates
-
Antidiabetic PPAR gamma-activator rosiglitazone reduces MMP-9 serum levels in type 2 diabetic patients with coronary artery disease.
Authors: Marx N, Froehlich J, Siam L, Ittner J, Wierse G, Schmidt A, Scharnagl H, Hombach V, Koenig W
Arterioscler. Thromb. Vasc. Biol., 2003-02-01;23(2):283-8.
Species: Human
Sample Types: Serum
FAQs
-
Can I use EDTA or citrate plasma as samples in Human Total MMP-3 Quantikine ELISA Kit (Catalog # DMP300)?
MMPs are a family of zinc and calcium dependent endopeptidases, and MMP3 catalytic domain contains a zinc binding site. Chelating agents such as EDTA and Citrate can sequester metal ions from the catalytic domain causing distruption of the MMP3 structure. MMP3 may be denatured as a result and may compromise measurements or detecteability in the assay.
Reviews for Human Total MMP-3 Quantikine ELISA Kit
Average Rating: 4 (Based on 1 Review)
Have you used Human Total MMP-3 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: