Human TGF-beta 2 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human TGF-beta2. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: TGF-beta 2
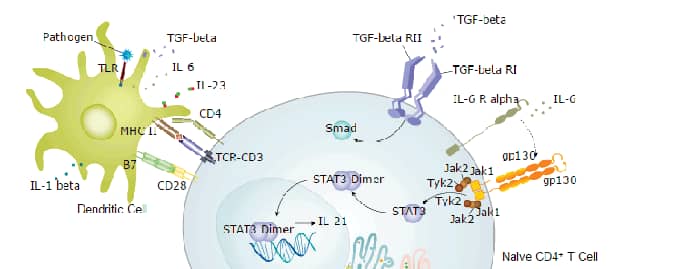
TGF-beta 2 is synthesized as a prepro-cytokine with a 19 amino acid (aa) signal sequence, a 283 aa pro-region, and a 112 aa mature segment (1-5). It dimerizes with formation of disulfide bonds between the 'pro' regions and disulfide bonds between the 'mature' regions. The mature region is 71% and 80% identical with human TGF-beta 1 and TGF-beta 3 (6, 7) and 97% identical with the corresponding mouse protein (8). After proteolytic cleavage of the disulfide-linked mature region, it remains hydrogen-bonded to the disulfide-linked prosegments (LAP or latencyassociated protein) (1, 2, 9). If secreted in this form, LAP keeps TGF-beta 2 in an inactive state until dissociation, caused by proteases, glycosidases, or extreme pH (2, 9). In many types of cells, an additional protein, latent TGF-beta binding protein (LTBP), is covalently linked to the LAP homodimer prior to secretion. LTBP, a 130 kDa cysteine-rich glycoprotein, creates a 235 kDa large latent complex that is secreted, most likely binding to the extracellular matrix (1, 9-11). The latency components are believed to act as natural antagonists of TGF-beta activity, to target TGF-beta to distinct tissues, and to maintain a reservoir of TGF-beta (1, 2, 12). On release from latency, active homodimeric TGF-beta can bind to cell-surface receptors or to other proteins, such as alpha 2-macroglobulin (13).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human TGF-beta 2 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
18
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Role of Betaglycan in TGF-beta Signaling and Wound Healing in Human Endometriotic Epithelial Cells and in Endometriosis
Authors: AN Mwaura, MA Riaz, JB Maoga, E Mecha, COA Omwandho, G Scheiner-B, I Meinhold-H, L Konrad
Biology, 2022-03-26;11(4):.
Species: Human
Sample Types: Cell Culture Supernates
-
Nucleoporin-93 reveals a common feature of aggressive breast cancers: robust nucleocytoplasmic transport of transcription factors
Authors: NB Nataraj, A Noronha, JS Lee, S Ghosh, HR Mohan Raju, A Sekar, B Zuckerman, M Lindzen, E Tarcitano, S Srivastava, M Selitrenni, I Livneh, D Drago-Garc, O Rueda, C Caldas, S Lev, T Geiger, A Ciechanove, I Ulitsky, R Seger, E Ruppin, Y Yarden
Cell Reports, 2022-02-22;38(8):110418.
Species: Human
Sample Types: Cell Culture Supernates
-
Latent TGFbeta-binding proteins regulate UCP1 expression and function via TGFbeta2
Authors: D Halbgebaue, J Roos, JB Funcke, H Neubauer, BS Hamilton, E Simon, EZ Amri, KM Debatin, M Wabitsch, P Fischer-Po, D Tews
Molecular Metabolism, 2021-09-01;53(0):101336.
Species: Human
Sample Types: Cell Culture Supernates
-
Csnk1a1 inhibition modulates the inflammatory secretome and enhances response to radiotherapy in glioma
Authors: G Liu, H Li, W Zhang, J Yu, X Zhang, R Wu, M Niu, X Liu, R Yu
Journal of Cellular and Molecular Medicine, 2021-07-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Regulation of Fibroblast Activation Protein by Transforming Growth Factor Beta-1 in Glioblastoma Microenvironment
Authors: E Krepela, Z Vanickova, P Hrabal, M Zubal, B Chmielova, E Balaziova, P Vymola, I Matrasova, P Busek, A Sedo
International Journal of Molecular Sciences, 2021-01-21;22(3):.
Species: Human
Sample Types: Cell Lysates
-
Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis
Authors: JH Ko, HJ Kim, HJ Jeong, HJ Lee, JY Oh
Cell Rep, 2020-03-17;30(11):3806-3820.e6.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered Decorin Biology in Proliferative Vitreoretinopathy: A Mechanistic and Cohort Study
Authors: G Begum, J O'Neill, R Chaudhary, K Blachford, DRJ Snead, M Berry, RAH Scott, A Logan, RJ Blanch
Invest. Ophthalmol. Vis. Sci., 2018-10-01;59(12):4929-4936.
Species: Human
Sample Types: Vitreous Humor
-
Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy
Authors: SE Logue, EP McGrath, P Cleary, S Greene, K Mnich, A Almanza, E Chevet, RM Dwyer, A Oommen, P Legembre, F Godey, EC Madden, B Leuzzi, J Obacz, Q Zeng, JB Patterson, R Jäger, AM Gorman, A Samali
Nat Commun, 2018-08-15;9(1):3267.
Species: Human
Sample Types: Cell Culture Supernates
-
The peritoneum is both a source and target of TGF-beta in women with endometriosis.
Authors: Young V, Brown J, Saunders P, Duncan W, Horne A
PLoS ONE, 2014-09-10;9(9):e106773.
Species: Human
Sample Types: Peritoneal Fluid
-
Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components.
Authors: Reeves S, Kolstad T, Lien T, Elliott M, Ziegler S, Wight T, Debley J
J Allergy Clin Immunol, 2014-05-27;134(3):663-670.e1.
Species: Human
Sample Types: Cell Culture Supernates
-
Primary culture of avian embryonic heart forming region cells to study the regulation of vertebrate early heart morphogenesis by vitamin A.
Authors: Cakstina I, Riekstina U, Boroduskis M, Nakurte I, Ancans J, Zile M, Muiznieks I
BMC Dev Biol, 2014-02-19;14(0):10.
Species: Chicken
Sample Types: Cell Culture Supernates
-
Activin A as a mediator of NK-dendritic cell functional interactions.
Authors: Seeger P, Bosisio D, Parolini S, Badolato R, Gismondi A, Santoni A, Sozzani S
J Immunol, 2014-01-06;192(3):1241-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts.
Authors: Hawinkels L, Paauwe M, Verspaget H, Wiercinska E, van der Zon J, van der Ploeg K, Koelink P, Lindeman J, Mesker W, ten Dijke P, Sier C
Oncogene, 2012-12-03;33(1):97-107.
Species: Human
Sample Types: Cell Culture Supernates
-
Glioblastoma-secreted factors induce IGFBP7 and angiogenesis by modulating Smad-2-dependent TGF-beta signaling.
Authors: Pen A, Moreno MJ, Durocher Y, Deb-Rinker P, Stanimirovic DB
Oncogene, 2008-08-18;27(54):6834-44.
Species: Human
Sample Types: Cell Culture Supernates
-
Opposite regulation of transforming growth factors-beta2 and -beta3 expression in the human endometrium.
Authors: Gaide Chevronnay HP, Cornet PB, Delvaux D, Lemoine P, Courtoy PJ, Henriet P, Marbaix E
Endocrinology, 2007-11-26;149(3):1015-25.
Species: Human
Sample Types: Tissue Homogenates
-
Transforming growth factor-beta1 suppresses airway hyperresponsiveness in allergic airway disease.
Authors: Alcorn JF, Rinaldi LM, Jaffe EF, van Loon M, Bates JH, Janssen-Heininger YM, Irvin CG
Am. J. Respir. Crit. Care Med., 2007-08-29;176(10):974-82.
Species: Mouse
Sample Types: BALF
-
Activation of transforming growth factor-beta by the integrin alphavbeta8 delays epithelial wound closure.
Authors: Neurohr C, Nishimura SL, Sheppard D
Am. J. Respir. Cell Mol. Biol., 2006-03-30;35(2):252-9.
Species: Human
Sample Types: Cell Culture Supernates
-
The selective estrogen receptor modulator arzoxifene and the rexinoid LG100268 cooperate to promote transforming growth factor beta-dependent apoptosis in breast cancer.
Authors: Rendi MH, Suh N, Lamph WW, Krajewski S, Reed JC, Heyman RA, Berchuck A, Liby K, Risingsong R, Royce DB, Williams CR, Sporn MB
Cancer Res., 2004-05-15;64(10):3566-71.
Species: Rat
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human TGF-beta 2 DuoSet ELISA
There are currently no reviews for this product. Be the first to review Human TGF-beta 2 DuoSet ELISA and earn rewards!
Have you used Human TGF-beta 2 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image