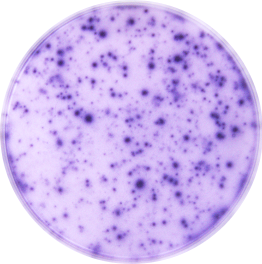
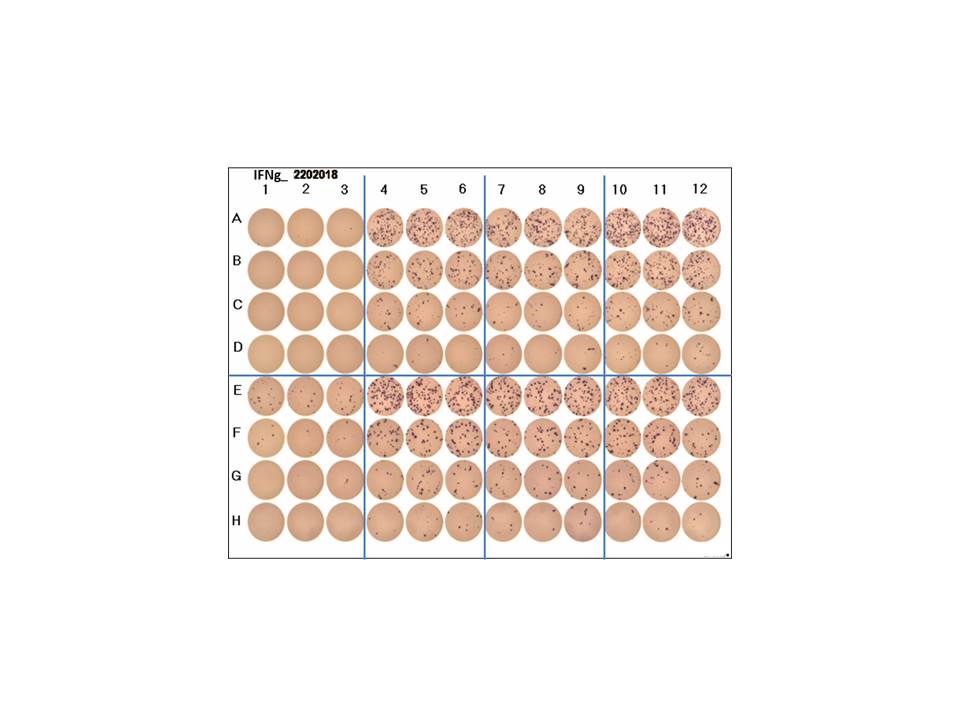
Human IFN-gamma ELISpot Kit Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
PRODUCT SUMMARY
ELISpot kits are highly sensitive, microplate-based assays for the detection of cytokine secreting cells. This kit is designed for the detection and enumeration of human IFN-gamma. Complete ELISpot kits are ready-to-run and require no assay development or refinement.
This ELISpot assay employs a capture antibody specific for human IFN-gamma, pre-coated onto a PVDF-backed microplate. Appropriately stimulated cells are pipetted directly into the wells and the immobilized antibody in the immediate vicinity of the secreting cells binds secreted human IFN-gamma. Following wash steps and incubation with a biotinylated detection antibody, alkaline-phosphatase conjugated to streptavidin is added. Unbound enzyme is subsequently removed by washing and a substrate solution (BCIP/NBT) is added. A blue-black colored precipitate forms at the sites of cytokine localization and appears as spots, with each individual spot representing an individual human IFN-gamma secreting cell. The spots can be counted with an automated ELISpot reader system or manually using a stereomicroscope.
PRODUCT FEATURES
- Detect and quantitate individual cells secreting human IFN-gamma
- High sensitivity - ELISpot assays can measure responses with frequencies well below 1 in 100,000 cells
- No in vitro expansion of cells required
- High-throughput - ELISpot assays use only a small number of primary cells
KIT CONTENTS
- Human IFN-gamma Microplate
- Biotinylated Detection Antibody
- Streptavidin conjugated to Alkaline Phosphatase
- Dilution Buffers
- Wash Buffer Concentrate
- BCIP/NBT Chromogen
- Human IFN-gamma Positive Control
OTHER REAGENTS REQUIRED
- Pipettes and pipette tips
- Deionized or distilled water
- Squirt bottle, manifold dispenser, or automated microplate washer
- 500 mL graduated cylinder
- 37 °C CO2 incubator
- Sterile culture media
- Dissection microscope or an automated ELISpot reader
Product Datasheets
Preparation and Storage
Background: IFN-gamma
IFN-gamma (Interferon-gamma) is the prototype proinflammatory cytokine and is produced by a variety of immune cells under inflammatory conditions, notably by T cells and NK cells. It plays a key role in host defense by promoting the development and activation of Th1 cells, chemoattraction and activation of monocytes and macrophages, upregulation of antigen presentation molecules, and immunoglobulin class switching in B cells. It also exhibits antiviral, antiproliferative, and apoptotic effects. In addition, IFN-gamma functions as an anti-inflammatory mediator by promoting the development of regulatory T cells and inhibiting Th17 cell differentiation. IFN-gamma dimers signal through a receptor complex of two IFN-gamma R1 and two IFN-gamma R2 subunits.
Citations for Human IFN-gamma ELISpot Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
28
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Nivolumab plus ipilimumab in advanced salivary gland cancer: a phase 2 trial
Authors: Vos, JL;Burman, B;Jain, S;Fitzgerald, CWR;Sherman, EJ;Dunn, LA;Fetten, JV;Michel, LS;Kriplani, A;Ng, KK;Eng, J;Tchekmedyian, V;Haque, S;Katabi, N;Kuo, F;Han, CY;Nadeem, Z;Yang, W;Makarov, V;Srivastava, RM;Ostrovnaya, I;Prasad, M;Zuur, CL;Riaz, N;Pfister, DG;Klebanoff, CA;Chan, TA;Ho, AL;Morris, LGT;
Nature medicine
Species: Human
Sample Types: Whole Cells
-
Vaccine-Induced T-Cell and Antibody Responses at 12 Months after Full Vaccination Differ with Respect to Omicron Recognition
Authors: F Mai, J Volzke, EC Reisinger, B Müller-Hil
Vaccines, 2022-09-19;10(9):.
Species: Human
Sample Types: Whole Cells
-
Heterogenous humoral and cellular immune responses with distinct trajectories post-SARS-CoV-2 infection in a population-based cohort
Authors: D Menges, KD Zens, T Ballouz, N Caduff, D Llanas-Cor, HE Aschmann, A Domenghino, C Pellaton, M Perreau, C Fenwick, G Pantaleo, CR Kahlert, C Münz, MA Puhan, JS Fehr
Nature Communications, 2022-08-18;13(1):4855.
Species: Human
Sample Types: Whole Cells
-
Higher SARS-CoV-2 Spike Binding Antibody Levels and Neutralization Capacity 6 Months after Heterologous Vaccination with AZD1222 and BNT162b2
Authors: B Müller-Hil, F Mai, M Müller, J Volzke, EC Reisinger
Vaccines, 2022-02-17;10(2):.
Species: Human
Sample Types: Whole Cells
-
FcgammaR engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity
Authors: V Mysore, X Cullere, J Mears, F Rosetti, K Okubo, PX Liew, F Zhang, I Madera-Sal, F Rosenbauer, RM Stone, JC Aster, UH von Andria, AH Lichtman, S Raychaudhu, TN Mayadas
Nature Communications, 2021-08-09;12(1):4791.
Species: Human
Sample Types: Whole Cell
-
Anti-Tat immunity defines CD4+ T-cell dynamics in people living with HIV on long-term cART
Authors: A Tripiciano, O Picconi, S Moretti, C Sgadari, A Cafaro, V Francavill, A Arancio, G Paniccia, M Campagna, MR Pavone-Cos, L Sighinolfi, A Latini, VS Mercurio, MD Pietro, F Castelli, A Saracino, C Mussini, GD Perri, M Galli, S Nozza, F Ensoli, P Monini, B Ensoli
EBioMedicine, 2021-04-07;0(0):103306.
Species: Human
Sample Types: Whole Cells
-
Contribution of endotoxin to Th17 bias in patients with non-alcoholic steatohepatitis
Authors: X Wang, D Ji, B Zhu, S Jiang, L Han, Y Wang, H Mai, S Xu, H Jiang, G Wang, Y Rong
Microb. Pathog., 2020-01-27;0(0):104009.
Species: Human
Sample Types: Whole Cells
-
The first clinical use of a recombinant Lactococcus lactis expressing human papillomavirus type 16 E7 oncogene oral vaccine: A phase I safety and immunogenicity trial in healthy women volunteers
Authors: AH Mohseni, S Taghinezha, H Keyvani
Mol. Cancer Ther., 2019-10-23;0(0):.
Species: Human
Sample Types: Whole Cells
-
Sensitive Detection and Analysis of Neoantigen-Specific T Cell Populations from Tumors and Blood
Authors: S Peng, JM Zaretsky, AHC Ng, W Chour, MT Bethune, J Choi, A Hsu, E Holman, X Ding, K Guo, J Kim, AM Xu, JE Heath, WJ Noh, J Zhou, Y Su, Y Lu, J McLaughlin, D Cheng, ON Witte, D Baltimore, A Ribas, JR Heath
Cell Rep, 2019-09-03;28(10):2728-2738.e7.
Species: Human
Sample Types: Whole Cells
-
Phase 1 Safety and Immunogenicity Trial of Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E6 Oncoprotein Vaccine
Authors: S Taghinezha, AH Mohseni, H Keyvani, MR Razavi
Mol Ther Methods Clin Dev, 2019-08-29;15(0):40-51.
Species: Human
Sample Types: Whole Cells
-
Immunogenic neoantigens derived from gene fusions stimulate T cell responses
Authors: W Yang, KW Lee, RM Srivastava, F Kuo, C Krishna, D Chowell, V Makarov, D Hoen, MG Dalin, L Wexler, R Ghossein, N Katabi, Z Nadeem, MA Cohen, SK Tian, N Robine, K Arora, H Geiger, P Agius, N Bouvier, K Huberman, K Vanness, JJ Havel, JS Sims, RM Samstein, R Mandal, J Tepe, I Ganly, AL Ho, N Riaz, RJ Wong, N Shukla, TA Chan, LGT Morris
Nat. Med., 2019-04-22;0(0):.
Species: Human
Sample Types: Whole Cells
-
A Potential Antitumor Effect of Dendritic Cells Fused with Cancer Stem Cells in Hepatocellular Carcinoma
Authors: YB Pang, J He, BY Cui, S Xu, XL Li, MY Wu, R Liang, Y Feng, X Guo, XH Zhang, XL Luo
Stem Cells Int, 2019-04-01;2019(0):5680327.
Species: Human
Sample Types: Whole Cells
-
Secretion of IFN-? Associated with Galectin-9 Production by Pleural Fluid Cells from a Patient with Extrapulmonary Tuberculosis
Authors: J Zhao, B Shiratori, H Chagan-Yas, M Matsumoto, T Niki, M Tanaka, Y Takahashi, O Usami, Y Ashino, T Hattori
Int J Mol Sci, 2017-06-28;18(7):.
Species: Human
Sample Types: Whole Cells
-
Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition.
Authors: Ren J, Liu X, Fang C, Jiang S, June C, Zhao Y
Clin Cancer Res, 2016-11-04;23(9):2255-2266.
Species: Human
Sample Types: Whole Cells
-
Comparative Multi-Donor Study of IFNgamma Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays.
Authors: Hagen J, Zimmerman R, Goetz C, Bonnevier J, Houchins J, Reagan K, Kalyuzhny A
Cells, 2015-02-11;4(1):84-95.
Species: Human
Sample Types: Whole Cells
-
Antigen-triggered interferon-gamma and interleukin-10 pattern in cured mucosal leishmaniasis patients is shaped during the active phase of disease.
Authors: Nogueira R, Gomes-Silva A, Bittar R, Silva Mendonca D, Amato V, da Silva Mattos M, Oliveira-Neto M, Coutinho S, Da-Cruz A
Clin Exp Immunol, 2014-09-01;177(3):679-86.
Species: Human
Sample Types: Whole Cells
-
Correlations between vaccinia-specific immune responses within a cohort of armed forces members.
Authors: Umlauf B, Ovsyannikova I, Haralambieva I, Kennedy R, Vierkant R, Pankratz V, Jacobson R, Poland G
Viral Immunol, 2011-09-29;24(5):415-20.
Species: Human
Sample Types: Whole Cells
-
Antibody-mediated depletion of lymphocyte-activation gene-3 (LAG-3(+) )-activated T lymphocytes prevents delayed-type hypersensitivity in non-human primates.
Authors: Poirier N, Haudebourg T, Brignone C, Dilek N, Hervouet J, Minault D, Coulon F, de Silly RV, Triebel F, Blancho G, Vanhove B
Clin. Exp. Immunol., 2011-02-24;164(2):265-74.
Species: Primate - Papio anubis (Olive Baboon)
Sample Types: Whole Cells
-
Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms.
Authors: Correale J, Farez M
J. Immunol., 2009-10-07;183(9):5999-6012.
Species: Human
Sample Types: Whole Cells
-
Interferon-gamma is induced in human peripheral blood immune cells in vitro by sodium stibogluconate/interleukin-2 and mediates its antitumor activity in vivo.
Authors: Fan K, Borden E, Yi T
J. Interferon Cytokine Res., 2009-08-01;29(8):451-60.
Species: Human
Sample Types: Whole Cells
-
Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals.
Authors: Derfuss T, Parikh K, Velhin S, Braun M, Mathey E, Krumbholz M, Kumpfel T, Moldenhauer A, Rader C, Sonderegger P, Pollmann W, Tiefenthaller C, Bauer J, Lassmann H, Wekerle H, Karagogeos D, Hohlfeld R, Linington C, Meinl E
Proc. Natl. Acad. Sci. U.S.A., 2009-04-28;106(20):8302-7.
Species: Human
Sample Types: Whole Cells
-
Epitope specificity and clonality of EBV-specific CTLs used to treat posttransplant lymphoproliferative disease.
Authors: McAulay KA, Haque T, Urquhart G, Bellamy C, Guiretti D, Crawford DH
J. Immunol., 2009-03-15;182(6):3892-901.
Species: Human
Sample Types: Whole Cells
-
Phase I therapeutic trial of the HIV-1 Tat protein and long term follow-up.
Authors: Longo O, Tripiciano A, Fiorelli V, Bellino S, Scoglio A, Collacchi B, Alvarez MJ, Francavilla V, Arancio A, Paniccia G, Lazzarin A, Tambussi G, Din CT, Visintini R, Narciso P, Antinori A, D'Offizi G, Giulianelli M, Carta M, Di Carlo A, Palamara G, Giuliani M, Laguardia ME, Monini P, Magnani M, Ensoli F, Ensoli B
Vaccine, 2009-02-07;27(25):3306-12.
Species: Human
Sample Types: Whole Cells
-
Impaired hypothalamic-pituitary-adrenal axis activity in patients with multiple sclerosis.
Authors: Ysrraelit MC, Gaitan MI, Lopez AS, Correale J
Neurology, 2008-12-09;71(24):1948-54.
Species: Human
Sample Types: Whole Cells
-
Human bronchial intraepithelial T cells produce interferon-gamma and stimulate epithelial cells.
Authors: Hirosako S, Goto E, Fujii K, Tsumori K, Hirata N, Tsumura S, Kamohara H, Kohrogi H
Clin. Exp. Immunol., 2008-11-26;155(2):266-74.
Species: Human
Sample Types: Whole Cells
-
Regulatory NK cells suppress antigen-specific T cell responses.
Authors: Deniz G, Erten G, Kucuksezer UC, Kocacik D, Karagiannidis C, Aktas E, Akdis CA, Akdis M
J. Immunol., 2008-01-15;180(2):850-7.
Species: Human
Sample Types: Whole Cells
-
Mitoxantrone as rescue therapy in worsening relapsing-remitting MS patients receiving IFN-beta.
Authors: Correale J, Rush C, Amengual A, Goicochea MT
J. Neuroimmunol., 2005-05-01;162(1):173-83.
Species: Human
Sample Types: Whole Cells
-
A multidonor ELISPOT study of IL-1 beta, IL-2, IL-4, IL-6, IL-13, IFN-gamma and TNF-alpha release by cryopreserved human peripheral blood mononuclear cells.
Authors: Bailey T, Stark S, Grant A, Hartnett C, Tsang M, Kalyuzhny A
J. Immunol. Methods, 2002-12-15;270(2):171-82.
Species: Human
Sample Types: Whole Cells
FAQs
No product specific FAQs exist for this product, however you may
View all ELISpot and FluoroSpot Kit FAQsReviews for Human IFN-gamma ELISpot Kit
Average Rating: 4.6 (Based on 11 Reviews)
Have you used Human IFN-gamma ELISpot Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Works really well as compared to kits from other companies. Easy to follow protocol.
The kit is easy to use and produced excellent results. Couple things that need to be added are: Information regarding storage conditions of the developed kit (spots); In my experience, the kit once developed is pretty stable and can be stored @ 4C in the dark for at least few weeks. The positive control for the kit produces a lawn rather than spots, something to keep in mind.