Human G-CSF Quantikine ELISA Kit Summary
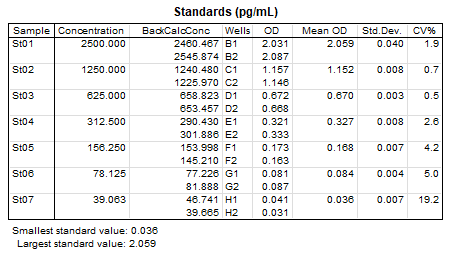
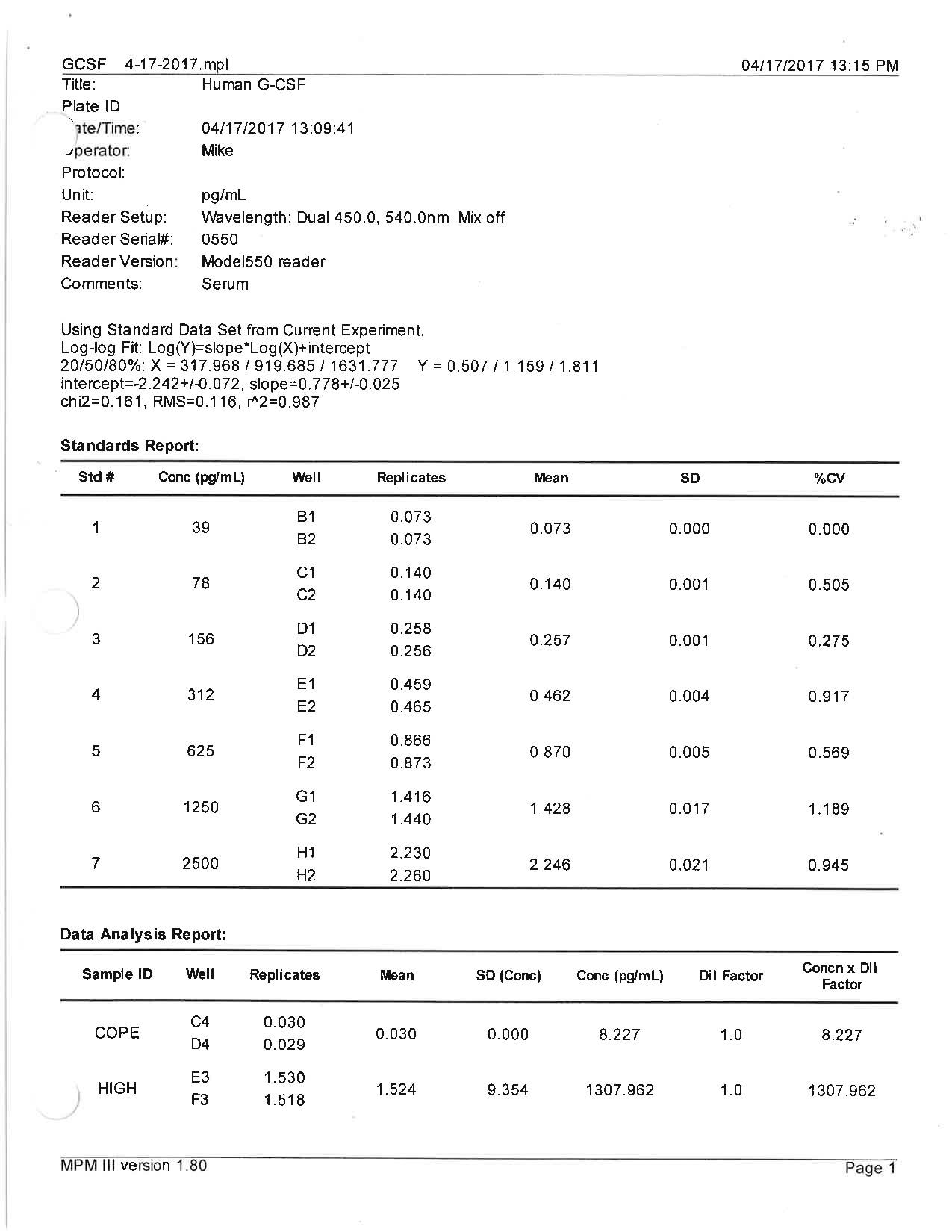
Sample Values
Serum/Plasma - Thirty-seven serum and plasma samples from apparently healthy volunteers were evaluated for the presence of human G-CSF in this assay. No medical histories were available for the donors used in this study. All samples measured less than the lowest human G-CSF standard, 39 pg/mL.| Condition | Day 1 (pg/mL) | Day 5 (pg/mL) |
| Unstimulated | 258 | 118 |
| Stimulated | 854 | 1139 |
Product Summary
Precision
Cell Culture Supernates
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 177 | 515 | 1047 | 143 | 818 | 1514 |
| Standard Deviation | 4.5 | 9.4 | 22 | 17 | 72 | 77.1 |
| CV% | 2.5 | 1.8 | 2.1 | 11.9 | 8.8 | 5.1 |
Serum, EDTA Plasma, Heparin Plasma, Citrate Plasma
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (pg/mL) | 280 | 827 | 1696 | 176 | 1094 | 2169 |
| Standard Deviation | 7.8 | 14.1 | 19.5 | 7.3 | 34.6 | 81.6 |
| CV% | 2.8 | 1.7 | 1.1 | 4.1 | 3.2 | 3.8 |
Recovery
The recovery of G-CSF spiked to three different levels throughout the range of the assay in various matrices was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=5) | 94 | 85-110 |
| Citrate Plasma (n=5) | 90 | 78-111 |
| EDTA Plasma (n=5) | 93 | 80-117 |
| Heparin Plasma (n=5) | 87 | 76-103 |
| Serum (n=5) | 88 | 75-106 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: G-CSF
Granulocyte-colony stimulating factor (G-CSF) is a 24-25 kDa monomeric glycoprotein that regulates the proliferation, differentiation, and activation of hematopoietic cells in the neutrophilic granulocyte lineage. Mature human G-CSF is a 178 amino acid (aa) O-glycosylated protein that contains two intrachain disulfide bridges. In humans, alternate splicing generates a second minor isoform with a 3 aa deletion. Mouse and human G-CSF share 76% aa sequence identity, and the two proteins show species cross-reactivity. G-CSF is produced by activated monocytes and macrophages, fibroblasts, endothelial cells, astrocytes, neurons, and bone marrow stroma cells. In addition, various tumor cells express G-CSF constitutively.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process twice for a total of 3 washes.
- Add 200 µL of Conjugate to each well.
- For Serum & Plasma Samples: Cover with a new plate sealer, and incubate at room temperature for 2 hours.
For Cell Culture Supernate Samples: Cover with a new plate sealer, and incubate at room temperature for 1 hour. - Aspirate and wash 3 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 20 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human G-CSF Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
45
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Protective Effects of Keratinocyte-Derived GCSF and CCL20 on UVB-Induced Melanocyte Damage
Authors: Jeayeng, S;Saelim, M;Muanjumpon, P;Buraphat, P;Kanchanapiboon, P;Sampattavanich, S;Panich, U;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
A Comparative Analysis of Innate Immune Responses and the Structural Characterization of Spike from SARS-CoV-2 Gamma Variants and Subvariants
Authors: Scovino, AM;Dahab, EC;Diniz-Lima, I;de Senna Silveira, E;Barroso, SPC;Cardoso, KM;Nico, D;Makhoul, GJ;da Silva-Junior, EB;Freire-de-Lima, CG;Freire-de-Lima, L;Fonseca, LMD;Valente, N;Nacife, V;Machado, A;Araújo, M;Vieira, GF;Pauvolid-Corrêa, A;Siqueira, M;Morrot, A;
Microorganisms
Species: Human
Sample Types: Cell Culture Supernates
-
Efficient differentiation of human neutrophils with recapitulation of emergency granulopoiesis in human G-CSF knockin humanized mice
Authors: R Ito, I Katano, IWH Kwok, LG Ng, M Ida-Tanaka, Y Ohno, Y Mu, H Morita, E Nishinaka, C Nishime, M Mochizuki, K Kawai, TH Chien, Z Yunqian, F Yiping, LH Hua, T Celhar, JK Yen Chan, T Takahashi, M Goto, T Ogura, R Takahashi, M Ito
Cell Reports, 2022-12-20;41(12):111841.
Species: Mouse
Sample Types: Plasma
-
Cytokine response over the course of COVID-19 infection in pregnant women
Authors: DB Rosen, EA Murphy, RS Gejman, A Capili, RL Friedlande, S Rand, KA Cagino, SM Glynn, KC Matthews, JM Kubiak, J Yee, M Prabhu, LE Riley, YJ Yang
Cytokine, 2022-04-25;154(0):155894.
Species: Human
Sample Types: Serum
-
Extension of human GCSF serum half-life by the fusion of albumin binding domain
Authors: FY Nikravesh, S Shirkhani, E Bayat, Y Talebkhan, E Mirabzadeh, M Sabzalinej, HAM Aliabadi, L Nematollah, YH Ardakani, S Sardari
Scientific Reports, 2022-01-13;12(1):667.
Species: Rat
Sample Types: Plasma
-
Conquering the cytokine storm in COVID-19-induced ARDS using placenta-derived decidua stromal cells
Authors: B Sadeghi, E Roshandel, A Pirsalehi, S Kazemi, G Sankanian, M Majidi, M Salimi, N Aghdami, H Sadrosadat, S Samadi Koc, F Alaeddini, O Ringden, A Hajifathal
Journal of Cellular and Molecular Medicine, 2021-10-10;0(0):.
Species: Human
Sample Types: Serum
-
The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males.
Authors: Tominaga T, Ikemura T, Yada K, Kanda K, Sugama K, Ma S, Choi W, Araya M, Huang J, Nakamura N, Suzuki K
Antioxidants (Basel), 2021-05-28;10(6):.
Species: Human
Sample Types: Urine
-
Cytokine Profiles of Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy with Regards to Radiation Pneumonitis Severity
Authors: BK Jeong, JH Kim, MH Jung, KM Kang, YH Lee
Journal of Clinical Medicine, 2021-02-11;10(4):.
Species: Human
Sample Types: Plasma
-
Prognostic significance of bone marrow FDG uptake in patients with gynecological cancer
Authors: K Shimura, S Mabuchi, N Komura, E Yokoi, K Kozasa, T Sasano, M Kawano, Y Matsumoto, T Watabe, M Kodama, K Hashimoto, K Sawada, J Hatazawa, T Kimura
Scientific Reports, 2021-01-26;11(1):2257.
Species: Human
Sample Types: Serum
-
Pretreatment tumor-related leukocytosis misleads positron emission tomography-computed tomography during lymph node staging in gynecological malignancies
Authors: S Mabuchi, N Komura, T Sasano, K Shimura, E Yokoi, K Kozasa, H Kuroda, R Takahashi, M Kawano, Y Matsumoto, H Kato, J Hatazawa, T Kimura
Nat Commun, 2020-03-13;11(1):1364.
Species: Human
Sample Types: Cell Culture Supernates
-
The ELISA Detectability and Potency of Pegfilgrastim Decrease in Physiological Conditions: Key Roles for Aggregation and Individual Variability
Authors: T Xie, H Fang, W Ouyang, P Angart, MJ Chiang, AA Bhirde, F Sheikh, P Lynch, AB Shah, SM Patil, K Chen, M Shen, C Agarabi, RP Donnelly, K Brorson, SJ Schrieber, KE Howard, SM Rogstad, DM Frucht
Sci Rep, 2020-02-12;10(1):2476.
Species: Human
Sample Types: Serum
-
Durable multitransgene expression in vivo using systemic, nonviral DNA delivery
Authors: C Handumrong, AL Ye, SA Chmura, L Soroceanu, M Mack, RJ Ice, R Thistle, M Myers, SJ Ursu, Y Liu, M Kashani-Sa, TD Heath, D Liggitt, DB Lewis, R Debs
Sci Adv, 2019-11-27;5(11):eaax0217.
Species: Mouse
Sample Types: Plasma
-
Multilevel defects in the hematopoietic niche in essential thrombocythemia
Authors: T Sun, M Ju, X Dai, H Dong, W Gu, Y Gao, R Fu, X Liu, Y Huang, W Liu, Y Chi, W Wang, H Li, Y Zhou, L Shi, R Yang, L Zhang
Haematologica, 2019-07-09;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
G-CSF partially mediates effects of sleeve gastrectomy on the bone marrow niche
Authors: Z Li, J Hardij, SS Evers, CR Hutch, SM Choi, Y Shao, BS Learman, KT Lewis, RL Schill, H Mori, DP Bagchi, SM Romanelli, KS Kim, E Bowers, C Griffin, RJ Seeley, K Singer, DA Sandoval, CJ Rosen, OA MacDougald
J. Clin. Invest., 2019-05-06;129(6):2404-2416.
Species: Human
Sample Types: Plasma
-
C3P3-G1: first generation of a eukaryotic artificial cytoplasmic expression system
Authors: PH Jaïs, E Decroly, E Jacquet, M Le Boulch, A Jaïs, O Jean-Jean, H Eaton, P Ponien, F Verdier, B Canard, S Goncalves, S Chiron, M Le Gall, P Mayeux, M Shmulevitz
Nucleic Acids Res., 2019-03-18;0(0):.
Species: Transgenic Hamster
Sample Types: Cell Culture Supernates
-
Stroma-derived IL-6, G-CSF and Activin-A mediated dedifferentiation of lung carcinoma cells into cancer stem cells
Authors: CFD Rodrigues, E Serrano, MI Patrício, MM Val, P Albuquerqu, J Fonseca, CMF Gomes, AJ Abrunhosa, A Paiva, L Carvalho, MF Botelho, L Almeida, IM Carreira, MC Alpoim
Sci Rep, 2018-08-01;8(1):11573.
Species: Human
Sample Types: Cell Culture Supernates
-
Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer
Authors: C Thålin, S Lundström, C Seignez, M Daleskog, A Lundström, P Henriksson, T Helleday, M Phillipson, H Wallén, M Demers
PLoS ONE, 2018-01-11;13(1):e0191231.
Species: Human
Sample Types: Plasma
-
Profiling of cytokines, chemokines and other soluble proteins as a potential biomarker in colorectal cancer and polyps
Authors: NA Johdi, L Mazlan, I Sagap, R Jamal
Cytokine, 2017-07-06;99(0):35-42.
Species: Human
Sample Types: Serum
-
Peptidoglycan accelerates granulopoiesis through a TLR2- and MyD88-dependent pathway
Authors: M Takehara, S Seike, T Takagishi, K Kobayashi, M Nagahama
Biochem. Biophys. Res. Commun., 2017-04-15;487(2):419-425.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhanced non-viral gene delivery by coordinated endosomal release and inhibition of ?-tubulin deactylase
Nucleic Acids Res, 2017-04-07;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Kinetics of circulating endothelial progenitor cells in patients undergoing carotid artery surgery
Authors: Günay Kalender
Ther Clin Risk Manag, 2016-12-14;12(0):1841-1847.
Species: Human
Sample Types: Plasma
-
Multimodal Regulation of NET Formation in Pregnancy: Progesterone Antagonizes the Pro-NETotic Effect of Estrogen and G-CSF
Authors: Stavros Giaglis
Front Immunol, 2016-12-05;7(0):565.
Species: Human
Sample Types: Serum
-
Regulation of Circulating Hematopoietic Stem/Progenitor Cells in Preterm Infants with Septicemia
Stem Cells Dev, 2016-10-04;0(0):.
Species: Human
Sample Types: Plasma
-
Human lung fibroblasts may modulate dendritic cell phenotype and function: results from a pilot in vitro study
Authors: O Freynet, J Marchal-So, F Jean-Louis, A Mailleux, B Crestani, P Soler, L Michel
Respir Res, 2016-04-04;17(1):36.
Species: Human
Sample Types: Cell Culture Supernates
-
LPS-Induced G-CSF Expression in Macrophages Is Mediated by ERK2, but Not ERK1.
Authors: Chang S, Lin S, Yang H, Chou Y, Gao J, Lu S
PLoS ONE, 2015-06-26;10(6):e0129685.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin 4, interleukin 6 and osteopontin-serological markers of head and neck malignancy in primary diagnostics: A pilot study.
Authors: Aderhold C, Grobschmidt G, Sauter A, Faber A, Hormann K, Schultz J
Oncol Lett, 2014-07-04;8(3):1112-1118.
Species: Human
Sample Types: Serum
-
Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer.
Authors: Kumar, J, Fraser, F W, Riley, C, Ahmed, N, McCulloch, D R, Ward, A C
Br J Cancer, 2013-11-12;110(1):133-45.
Species: Human
Sample Types: Cell Culture Supernates
-
Randomized trial and pharmacokinetic study of pegfilgrastim versus filgrastim after dose-intensive chemotherapy in young adults and children with sarcomas.
Authors: Fox E, Widemann BC, Hawkins DS, Jayaprakash N, Dagher R, Aikin AA, Bernstein D, Long L, Mackall C, Helman L, Steinberg SM, Balis FM
Clin. Cancer Res., 2009-11-17;15(23):7361-7.
Species: Human
Sample Types: Serum
-
Age-dependent mobilization of circulating endothelial progenitor cells in infants and young children undergoing cardiac surgery with cardiopulmonary bypass.
Authors: Sun Y, Yi D, Wang Y, Zheng R, Sun G, Wang J, Liu Y, Ren J, Wang Y, Zhang S, Gu C, Pei J
Cytokine, 2009-07-24;47(3):206-13.
Species: Human
Sample Types: Plasma
-
Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs.
Authors: Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, Dunn CE, Davies ZA, Moss RB, Herzenberg LA, Herzenberg LA, Tirouvanziam R
Proc. Natl. Acad. Sci. U.S.A., 2009-03-17;106(14):5779-83.
Species: Human
Sample Types: Plasma
-
Novel immunocompetent murine tumor model for evaluation of conditionally replication-competent (oncolytic) murine adenoviral vectors.
Authors: Robinson M, Li B, Ge Y, Ko D, Yendluri S, Harding T, VanRoey M, Spindler KR, Jooss K
J. Virol., 2009-02-04;83(8):3450-62.
Species: Human
Sample Types: Cell Culture Supernates
-
Hematopoietic progenitor cell mobilization and harvesting in children with malignancies: do the advantages of pegfilgrastim really translate into clinical benefit?
Authors: Merlin E, Zohar S, Jérôme C, Veyrat-Masson R, Marceau G, Paillard C, Auvrignon A, Le Moine P, Gandemer V, Sapin V, Halle P, Boiret-Dupre N, Chevret S, Demeocq F, Dubray C, Kanold J
Bone Marrow Transplant., 2008-12-22;43(12):919-25.
Species: Human
Sample Types: Serum
-
Staphylococcus aureus in nasal lavage and biopsy of patients with chronic rhinosinusitis.
Authors: Niederfuhr A, Kirsche H, Deutschle T, Poppert S, Riechelmann H, Wellinghausen N
Allergy, 2008-10-01;63(10):1359-67.
Species: Human
Sample Types: Nasal Lavage Fluid
-
Does granulocyte colony-stimulating factor ameliorate the proinflammatory response in human meningococcal septic shock?
Authors: Rojahn A, Brusletto B, Ovstebo R, Haug KB, Kierulf P, Brandtzaeg P
Crit. Care Med., 2008-09-01;36(9):2583-9.
Species: Human
Sample Types: Plasma
-
Isolation and characterization of CD146+ multipotent mesenchymal stromal cells.
Authors: Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M
Exp. Hematol., 2008-05-27;36(8):1035-46.
Species: Human
Sample Types: Cell Culture Supernates
-
Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide.
Authors: Dower K, Ellis DK, Saraf K, Jelinsky SA, Lin LL
J. Immunol., 2008-03-01;180(5):3520-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts.
Authors: Hatfield K, Ryningen A, Corbascio M, Bruserud O
Int. J. Cancer, 2006-11-15;119(10):2313-21.
Species: Human
Sample Types: Cell Culture Supernates
-
Is granulocyte colony-stimulating factor level predictive for human IVF outcome?
Authors: Salmassi A, Schmutzler AG, Schaefer S, Koch K, Hedderich J, Jonat W, Mettler L
Hum. Reprod., 2005-05-12;20(9):2434-40.
Species: Human
Sample Types: Follicular Fluid, Serum
-
Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases.
Authors: Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, Tamassia N, Pieropan S, Biasi D, Sbarbati A, Sozzani S, Bambara L, Cassatella MA
Blood, 2004-09-09;105(2):830-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Enhanced circulating half-life and hematopoietic properties of a human granulocyte colony-stimulating factor/immunoglobulin fusion protein.
Authors: Cox GN, Smith DJ, Carlson SJ, Bendele AM, Chlipala EA, Doherty DH
Exp. Hematol., 2004-05-01;32(5):441-9.
Species: Human
Sample Types: Plasma
-
Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis.
Authors: Lamming CE, 175, Augustin L, Blackstad M, Lund TC, Hebbel RP, Verfaillie CM
2003-03-01;111(6):811-9.
Species: Human
Sample Types: Serum
-
Retroviral interleukin 1alpha gene transfer in bone marrow stromal cells in a primate model: induction of myelopoiesis stimulation.
Authors: de Revel T, Becard N, Sorg T, Rousseau S, Spano JP, Thiebot H, Methali M, Gras G, Le Grand R, Dormont D
Br. J. Haematol., 2002-09-01;118(3):875-84.
Species: Primate - Macaca fascicularis (Crab-eating Monkey or Cynomolgus Macaque)
Sample Types: Cell Culture Supernates
-
Distinct hematopoietic support by two human stromal cell lines.
Authors: Loeuillet C, Bernard G, Remy-Martin J, Saas P, Herve P, Douay L, Chalmers D
Exp. Hematol., 2001-06-01;29(6):736-45.
Species: Human
Sample Types: Cell Culture Supernates
-
Glioblastoma causing granulocytosis by secretion of granulocyte-colony-stimulating factor.
Authors: Hintzen RQ, Voormolen J, Sonneveld P, van Duinen SG
Neurology, 2000-01-11;54(1):259-61.
Species: Human
Sample Types: CSF
-
Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF.
Authors: Fadok, V A, Bratton, D L, Konowal, A, Freed, P W, Westcott, J Y, Henson, P M
J Clin Invest, 1998-02-15;101(4):890-8.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human G-CSF Quantikine ELISA Kit
Average Rating: 4.5 (Based on 4 Reviews)
Have you used Human G-CSF Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: