Human CMG-2/ANTXR2 Antibody Summary
Gln34-Asn317
Accession # P58335
Applications
This antibody functions as an ELISA detection antibody when paired with Mouse Anti-Human CMG‑2/ANTXR2 Monoclonal Antibody (Catalog # MAB29401).
This product is intended for assay development on various assay platforms requiring antibody pairs.
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of CMG‑2/ANTXR2 in Human Monocytes by Flow Cytometry. Human whole blood monocytes were stained with Human CMG-2/ANTXR2 Antigen Affinity-purified Polyclonal Antibody (Catalog # AF2940, filled histogram) or control antibody (Catalog # AB-108-C, open histogram), followed by Phycoerythrin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0107).
 View Larger
View Larger
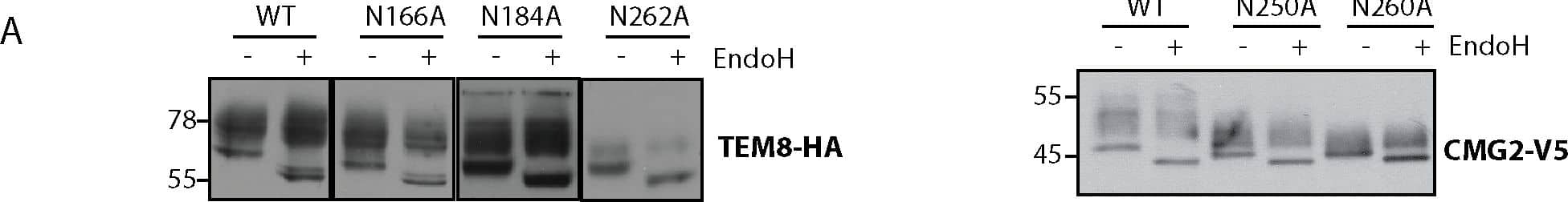
Detection of Human CMG-2/ANTXR2 by Western Blot Number and localization of glycan sidechains determine trafficking efficiency of TEM8 and CMG2.A) Endoglycosidase H (EndoH) treatment on TEM8 and CMG2 single mutants. HeLa cells were transfected for 48h with the respective cDNAs. 40 μg of cell extracts were treated or not with EndoH as described before. Samples were analyzed by SDS-PAGE and Western Blotting. B) Quantification of surface biotinylation experiments to determine amount of TEM8 at the cell surface. All mutants were corrected for their expression levels and then normalized to WT, which was set at 100%. Errors represent standard deviation. Statistics were calculated using an unpaired t-test. n ≥ 3. * p≤0.05, ** p≤0.01, *** p≤0.001 C) Representative Western Blots of surface biotinylation. HeLa cells were transfected 48h with the respective cDNAs. Proteins at the cell surface were labeled with biotin, immunoprecipitated with streptavidin beads and blotted against TEM8-HA. D) Quantification of surface biotinylation experiments to determine amount of CMG2 at the cell surface. All mutants were corrected for their expression levels and then normalized to WT, which was set at 100%. Errors represent standard deviation. Statistics were calculated using an unpaired t-test. n ≥ 3. * p≤0.05, ** p≤0.01, *** p≤0.001 E) Representative Western Blots of surface biotinylation. HeLa cells were transfected 48h with the respective cDNAs. Proteins at the cell surface were labeled with biotin, immunoprecipitated with streptavidin beads and blotted against CMG2-V5. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human CMG-2/ANTXR2 by Western Blot Number and localization of glycan sidechains determine trafficking efficiency of TEM8 and CMG2.A) Endoglycosidase H (EndoH) treatment on TEM8 and CMG2 single mutants. HeLa cells were transfected for 48h with the respective cDNAs. 40 μg of cell extracts were treated or not with EndoH as described before. Samples were analyzed by SDS-PAGE and Western Blotting. B) Quantification of surface biotinylation experiments to determine amount of TEM8 at the cell surface. All mutants were corrected for their expression levels and then normalized to WT, which was set at 100%. Errors represent standard deviation. Statistics were calculated using an unpaired t-test. n ≥ 3. * p≤0.05, ** p≤0.01, *** p≤0.001 C) Representative Western Blots of surface biotinylation. HeLa cells were transfected 48h with the respective cDNAs. Proteins at the cell surface were labeled with biotin, immunoprecipitated with streptavidin beads and blotted against TEM8-HA. D) Quantification of surface biotinylation experiments to determine amount of CMG2 at the cell surface. All mutants were corrected for their expression levels and then normalized to WT, which was set at 100%. Errors represent standard deviation. Statistics were calculated using an unpaired t-test. n ≥ 3. * p≤0.05, ** p≤0.01, *** p≤0.001 E) Representative Western Blots of surface biotinylation. HeLa cells were transfected 48h with the respective cDNAs. Proteins at the cell surface were labeled with biotin, immunoprecipitated with streptavidin beads and blotted against CMG2-V5. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
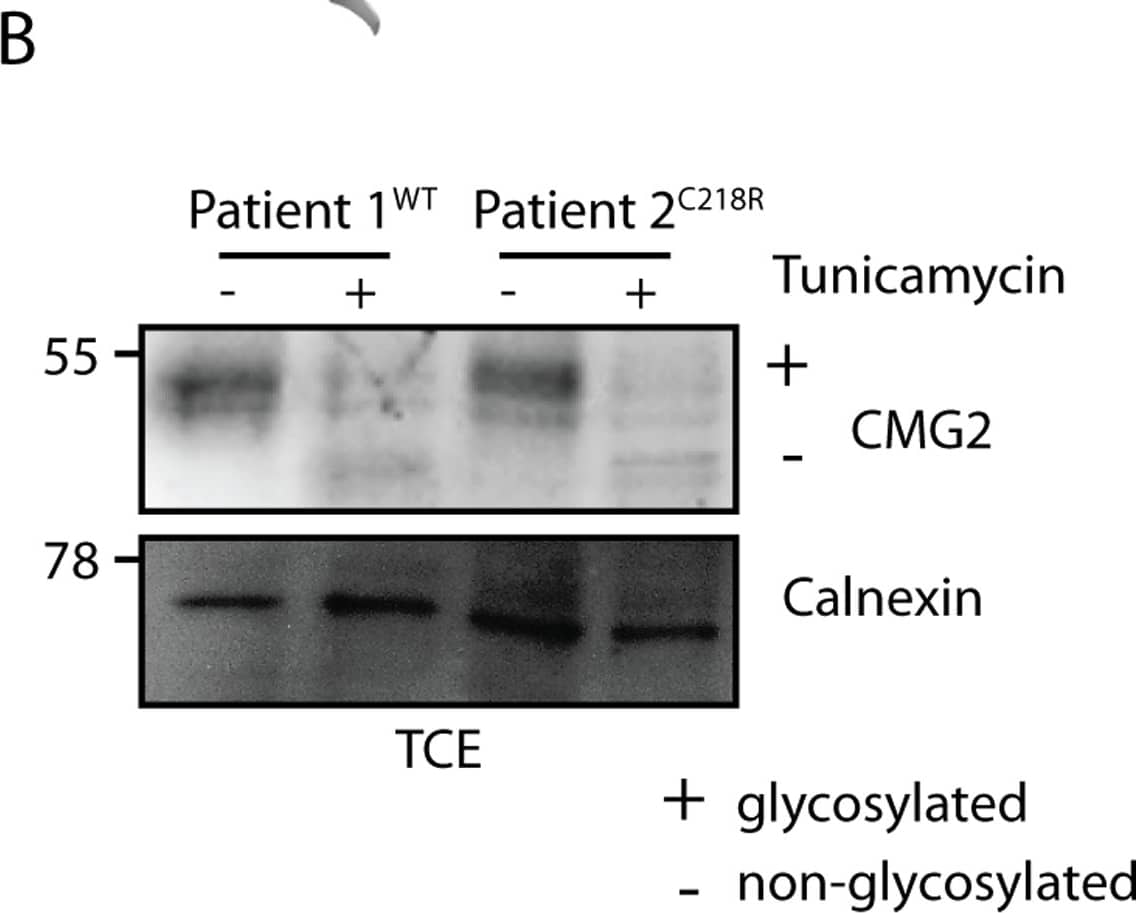
Detection of Human CMG-2/ANTXR2 by Immunoprecipitation Glycosylation acts as a buffer for CMG2 ectodomain mutations.A) Graphic showing the disulfide bridge C39-C218 (blue) present in CMG2 WT B) Fibroblast cells were treated or not with tunicamycin for 16h and TCE were analyzed by SDS-PAGE and Western Blot. Representative Western Blot with control fibroblasts and patient fibroblasts. Calnexin serves as a loading control. C) Quantification of total protein levels. CMG2 levels are normalized to WT protein level without tunicamycin treatment, which was set to 100%. Statistics were calculated using an unpaired t-test. Errors represent standard deviation. n ≥ 3. * p≤0.05 Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
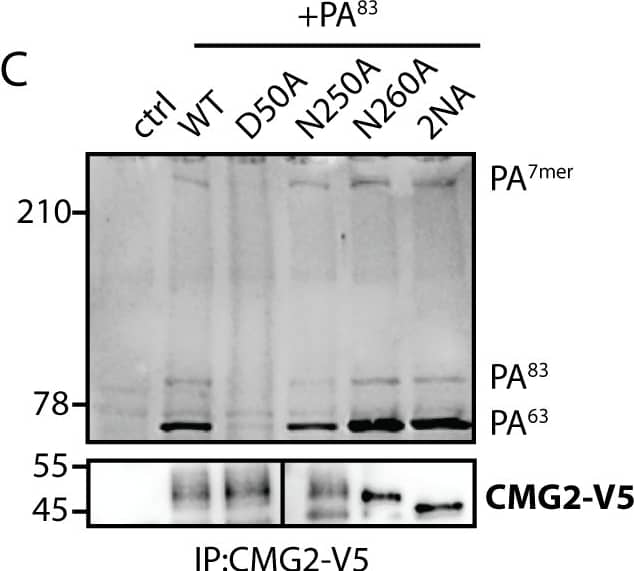 View Larger
View Larger
Detection of Human CMG-2/ANTXR2 by Immunoprecipitation Loss of glycosylation affects binding of Anthrax toxin to TEM8 but not to CMG2.A and C) HeLa cells were transfected for 48h with the respective cDNAs. Cells were treated for 1h at 4°C with 500 ng/ml PA83 and shifted to 37°C for 10 min to induce cleavage and heptamerization. Immunoprecipitates against TEM8-HA/CMG2-V5 were analyzed by SDS-PAGE and Western Blotting against PA and TEM8-HA/CMG2-V5. Control cells are non-transfected. D50A is a binding deficient mutant that serves as a negative control. B and D) HeLa cells were transfected for 48h with the respective cDNAs. Cells were lysed and incubated for 1h at 4°C with 1 μg/ml PA83. Immunoprecipitates against TEM8-HA/CMG2-V5 were analyzed by SDS-PAGE and Western Blotting against PA and TEM8-HA/CMG2-V5. Control cells are non-transfected. D50A is a binding deficient mutant that serves as a negative control. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
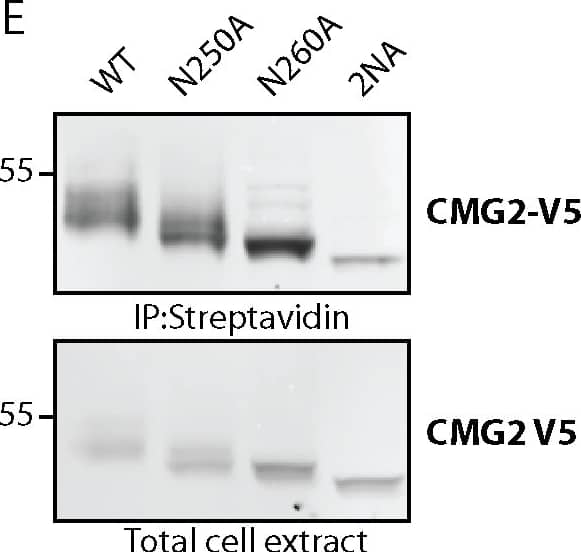
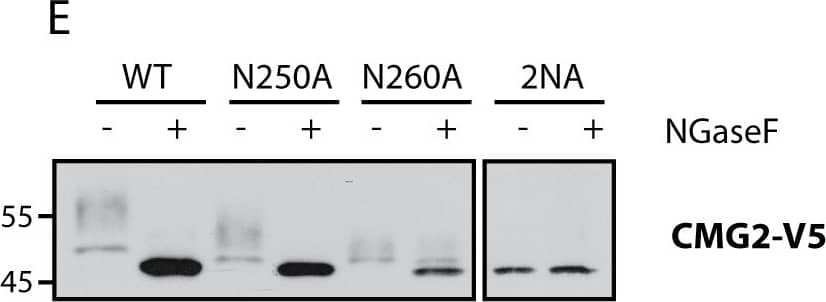
Detection of Human CMG-2/ANTXR2 by Immunoprecipitation CMG2 and TEM8 can undergo N-glycosylation on all predicted sites.A) Graphics depicting glycosylation sites on TEM8 and CMG2. Sites in red are unique to the respective proteins, N260 in CMG2 (yellow) corresponds to N262 in TEM8. B) Expression of TEM8 glycosylation mutants in HeLa cells. Cells were transfected for 48h with the respective cDNAs. Expression was analyzed by SDS-PAGE and Western Blotting. C) Expression of all CMG2 glycosylation mutants in HeLa cells. Cells were transfected for 48h with the respective cDNAs. Expression was analyzed by SDS-PAGE and Western Blotting. D) Endoglycosidase F (NGaseF) treatment on TEM8 glycosylation mutants. 40 μg of cell extracts were treated or not with NGaseF and analyzed by SDS-PAGE and Western Blotting. E) Endoglycosidase F (NGaseF) treatment on CMG2 glycosylation mutants. 40 μg of cell extracts were treated or not with NGaseF and analyzed by SDS-PAGE and Western Blotting. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
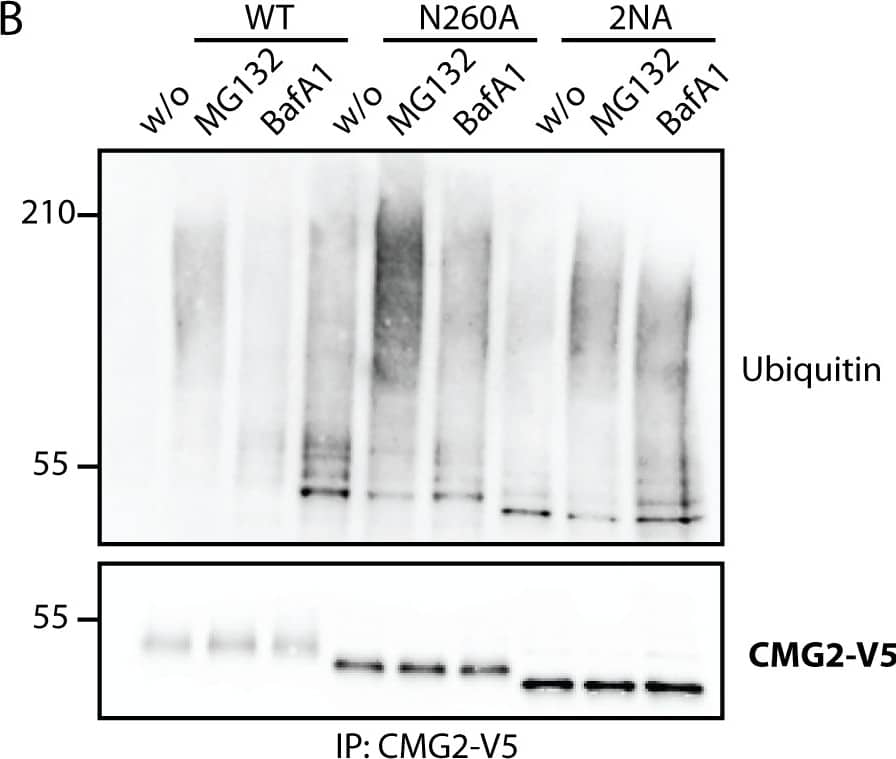
Detection of Human CMG-2/ANTXR2 by Western Blot Non-glycosylated TEM8 is an ER quality control and ERAD substrate.A) HeLa cells were transfected for 48h with the respective cDNAs. Cells were treated or not with MG132, an inhibitor of the proteasome or Bafilomycin A1, a drug preventing endosomal acidification and thus lysosomal degradation. Immunoprecipitates against TEM8-HA were analyzed by SDS-PAGE and Western Blotting against Ubiquitin and TEM8-HA. B) HEK cells stably expressing CMG2 under the control of a tetracycline inducible promotor were induced for 24h with 0.1μg/ml doxycycline. Cells were treated or not with MG132 or Bafilomycin A1. Immunoprecipitates against CMG2-V5 were analyzed by SDS-PAGE and Western Blotting against Ubiquitin and CMG2-V5. C) HeLa cells were treated or not with tunicamycin, an antibiotic blocking the co-translational transfer of glycan sidechains in the ER by blocking the oligosaccharyltransferase (OST) for 16h. Surface proteins were labeled with biotin and immunoprecipitates against streptavidin were analysed for TEM8 or Calnexin as a negative control. D) RpeI cells were treated or not with tunicamycin for 16h. Surface proteins were labeled with biotin and immunoprecipitates against streptavidin were analysed for CMG2 or Calnexin as a negative control. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/25781883), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CMG-2/ANTXR2
Capillary Morphogenesis Gene-2 (CMG-2) is a widely expressed anthrax toxin receptor (ATR) family protein (1‑3). CMG-2 is a 55 kDa type I transmembrane (TM) protein that contains a 33 amino acid (aa) signal sequence, a 284 aa extracellular domain (ECD), a 24 aa TM segment, and a 147 aa cytoplasmic domain. There are three additional isoforms. Isoforms 4 shows a 12 aa insertion in the cytoplasmic region; isoform 2 shows a 103 aa deletion in the ECD; and isoform 3 is a truncated, 20 kDa, 289 aa soluble form. The main functional domain of CMG-2 is an extracellular integrin-like von Willebrand factor type A (VWA) domain with a metal ion dependent adhesion site (MIDAS). This domain adheres selectively to collagen type IV and laminin (1‑5). CMG-2 isoform 2 is induced in HUVEC as they undergo capillary formation in collagen matrices in vitro (3). CMG-2 is mutated in juvenile hyaline fibromatosis and infantile systemic hyalinosis disorders, and several of these mutations result in loss of laminin binding (6). CMG-2 and the related protein ATR/TEM8 serve as receptors for the protective antigen (PA) of Bacillus Anthracis (1, 2). After binding the VWA domain, PA undergoes furin-type cleavage, forms a heptameric receptor/PA pre-pore and binds LF or EF toxin subunits (5, 7, 8). Transport to low pH endosomes, which requires CMG-2 ubiquitination and interaction with the LDL receptor related protein LRP6 (9, 10), allows PA pore formation and release of toxin to the cytoplasm (10, 11). Soluble CMG-2 VWA domain acts as a dummy receptor that can protect cultured cells from anthrax intoxication (2). Within the extracellular region, human CMG-2 shares 84%, 81%,89% and 93% amino acid sequence homology with mouse, rat, bovine, and canine CMG-2, respectively. CMG-2 VWA domain also shares 60% aa identity with ATR/TEM8.
- Scobie, H.M. and J.A.T. Young (2005) Curr. Opin. Microbiol. 8:106.
- Scobie, H.M. et al. (2003) Proc. Natl. Acad. Sci. USA 100:5170.
- Bell, S.E. et al. (2001) J. Cell Sci. 114:2755.
- Lacy, D.B. et al. (2004) Proc. Natl. Acad. Sci. USA 101:6367.
- Santelli, E. et al. (2004) Nature 430:905.
- Dowling, O. et al. (2003) Am. J. Hum. Genet. 73:957.
- Wigelsworth, D.J. et al. (2004) J. Biol. Chem. 279:23349.
- Go, M.Y. et al. (2006) J. Mol. Biol. 360:145.
- Abrami, L. et al. (2006) J. Cell Biol. 172:309.
- Wei, W. et al. (2006) Cell 124:1141.
- Lacy, D.B. et al. (2004) Proc. Natl. Acad. Sci. USA 101:13147.
Product Datasheets
Citations for Human CMG-2/ANTXR2 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
8
Citations: Showing 1 - 8
Filter your results:
Filter by:
-
Hyaline Fibromatosis Syndrome inducing mutations in the ectodomain of anthrax toxin receptor 2 can be rescued by proteasome inhibitors
Authors: Julie Deuquet, Ekkehart Lausch, Nicolas Guex, Laurence Abrami, Suzanne Salvi, Asvin Lakkaraju et al.
EMBO Molecular Medicine
-
The Capillary Morphogenesis Gene 2 Triggers the Intracellular Hallmarks of Collagen VI-Related Muscular Dystrophy
Authors: E Castroflor, AJ Pérez Bern, A López-Márq, C Badosa, P Loza-Alvar, M Roldán, C Jiménez-Ma
International Journal of Molecular Sciences, 2022-07-11;23(14):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: STED Microscopy, Western Blot -
Differential dependence on N-glycosylation of anthrax toxin receptors CMG2 and TEM8.
Authors: Friebe S, Deuquet J, van der Goot F
PLoS ONE, 2015-03-17;10(3):e0119864.
Species: Human
Sample Types: Whole Cells
Applications: Immunoprecipitation -
Therapeutic potential of capillary morphogenesis gene 2 extracellular vWA domain in tumourrelated angiogenesis.
Authors: Ye L, Sun P, Sanders A, Martin T, Lane J, Mason M, Jiang W
Int J Oncol, 2014-07-03;45(4):1565-73.
Species: Human
Sample Types: Protein
Applications: Western Blot -
Capillary morphogenesis gene 2 regulates adhesion and invasiveness of prostate cancer cells.
Authors: Ye, Lin, Sanders, Andrew J, Sun, Ping-Hui, Mason, Malcolm, Jiang, Wen G
Oncol Lett, 2014-04-04;7(6):2149-2153.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Bacillus anthracis lethal toxin reduces human alveolar epithelial barrier function.
Authors: Langer, Marybeth, Duggan, Elizabet, Booth, John Lel, Patel, Vineet I, Zander, Ryan A, Silasi-Mansat, Robert, Ramani, Vijay, Veres, Tibor Zo, Prenzler, Frauke, Sewald, Katherin, Williams, Daniel M, Coggeshall, Kenneth, Awasthi, Shanjana, Lupu, Florea, Burian, Dennis, Ballard, Jimmy Da, Braun, Armin, Metcalf, Jordan P
Infect Immun, 2012-10-01;80(12):4374-87.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: Flow Cytometry, Western Blot -
Resistance of human alveolar macrophages to Bacillus anthracis lethal toxin.
Authors: Wu W, Mehta H, Chakrabarty K, Booth JL, Duggan ES, Patel KB, Ballard JD, Coggeshall KM, Metcalf JP
J. Immunol., 2009-10-07;183(9):5799-806.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Ubiquitin-dependent folding of the Wnt signaling coreceptor LRP6
Authors: Elsa Perrody, Laurence Abrami, Michal Feldman, Beatrice Kunz, Sylvie Urbé, F Gisou van der Goot
eLife
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human CMG-2/ANTXR2 Antibody
There are currently no reviews for this product. Be the first to review Human CMG-2/ANTXR2 Antibody and earn rewards!
Have you used Human CMG-2/ANTXR2 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
