Wnt Signaling Pathways: beta-Catenin-dependent Wnt Signaling
Click one of the boxes to see how the presence (+) or absence (-) of the factors listed affects the Wnt/beta-Catenin-dependent signaling pathway.
Antagonists
Antagonists
USAG1
SOST
Draxin
IGFBP-4
USAG1
SOST
Draxin
IGFBP-4
Antagonists
Antagonists
WIF
Notum
WIF
Notum
Destruction Complex
Destruction Complex
Proteasomal
Degradation
Proteasomal
Degradation
Target Genes
Target Genes
ZNRF3
ZNRF3
Membrane
Clearance of
RNF43/ZNRF3
Membrane
Clearance of
RNF43/ZNRF3
Recruitment
of Axin to LRP-5/6
Recruitment
of Axin to LRP-5/6
Endocytosis of the
Wnt-Receptor Complex
Endocytosis of the
Wnt-Receptor Complex
ZNRF3
ZNRF3
Stabilization of
Wnt Receptors on the
Plasma Membrane
Stabilization of
Wnt Receptors on the
Plasma Membrane
Stabilization of
Newly Synthesized
beta-Catenin
Stabilization of
Newly Synthesized
beta-Catenin
Sequestration of GSK-3
beta Compromises beta-Catenin
Phosphorylation
Sequestration of GSK-3
beta Compromises beta-Catenin
Phosphorylation
Endosome
Endosome
Lysosomal Degradation
Lysosomal Degradation
Recycling
Recycling
ZNRF3
ZNRF3
Wnt Receptor
Endocytosis
Wnt Receptor
Endocytosis
Degradation
of Wnt
Receptors
Degradation
of Wnt
Receptors
Deubiquitination
and Recycling
Deubiquitination
and Recycling
Attenuation
of beta-Catenin-
Dependent
Wnt Signaling
Attenuation
of beta-Catenin-
Dependent
Wnt Signaling
Overview of Wnt Signaling
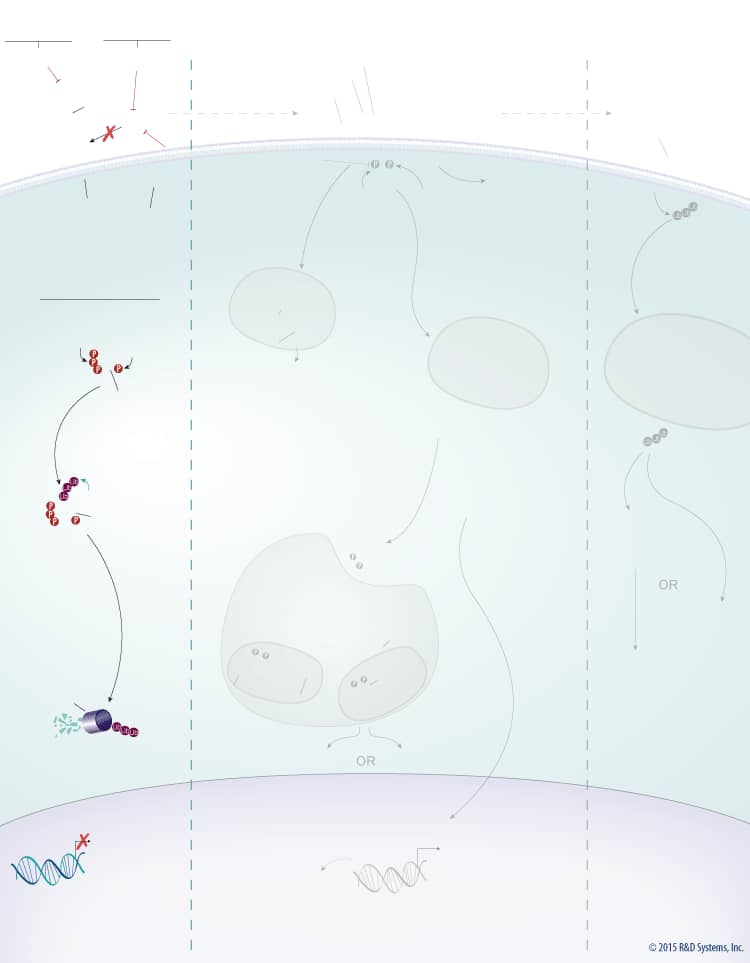
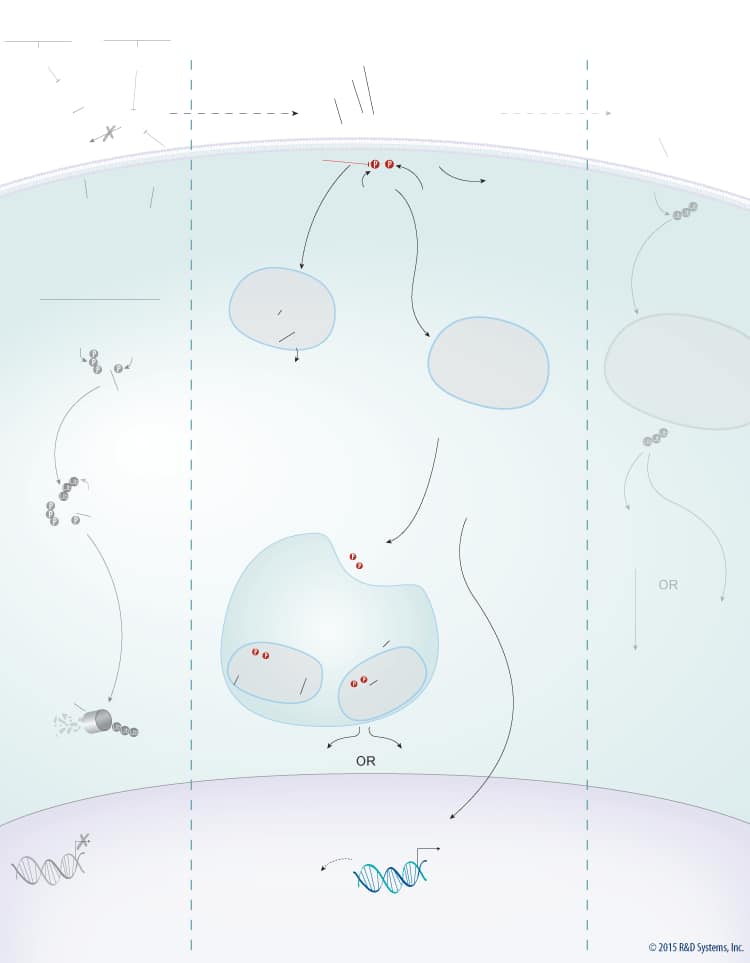
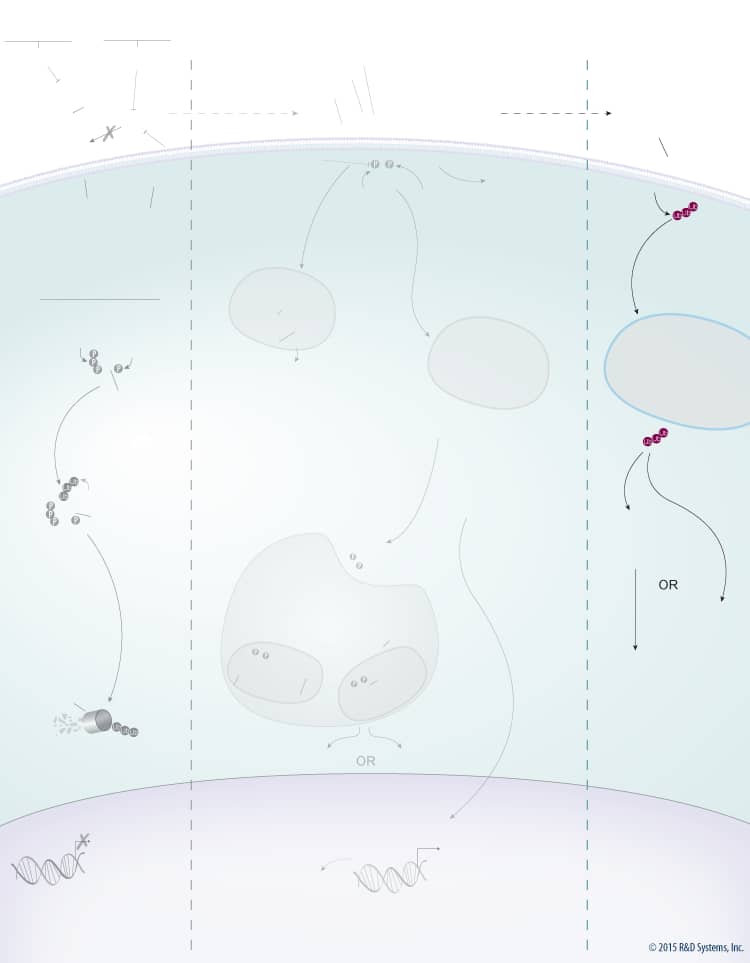
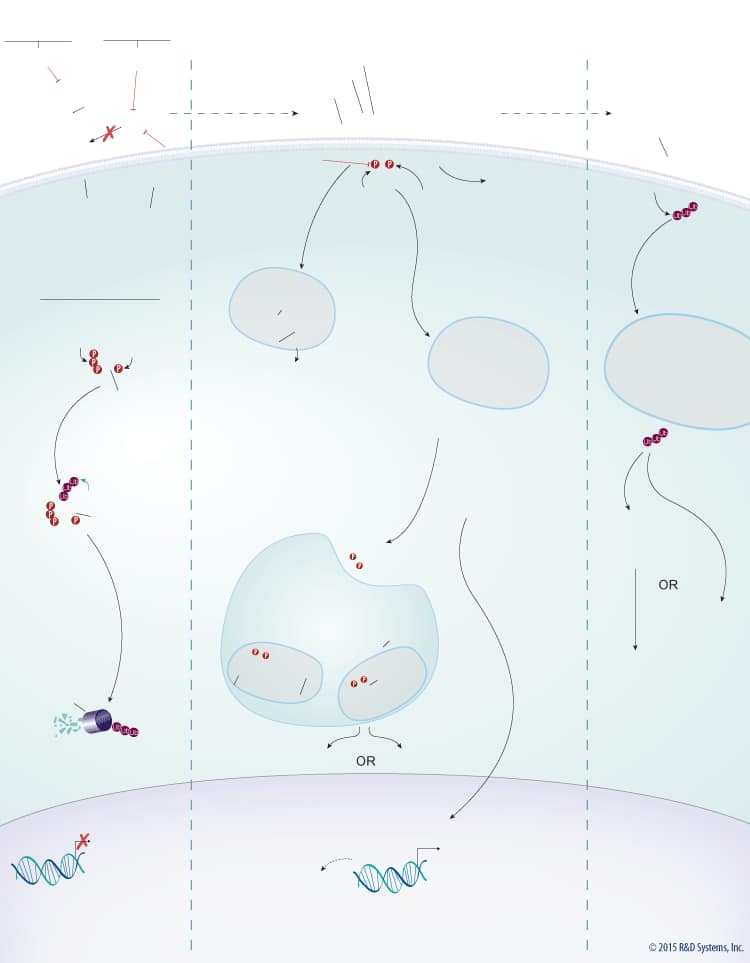
Wnts are a large family of secreted glycoproteins that play a central role in embryonic development, differentiation, cell motility, cell proliferation, and adult tissue homeostasis. Wnt signaling can activate beta-Catenin-dependent TCF/LEF transcription and at least two well-characterized beta-Catenin-independent pathways, the planar cell polarity (PCP) pathway and the Wnt/Ca2+ pathway. The beta-Catenin-dependent signaling pathway is initiated by association of Wnt with the Frizzled and LRP-5/6 receptors. This association activates Dishevelled, which subsequently recruits the Axin protein complex (Axin, APC, CK1, GSK-3 beta) to the receptor. Two kinases present in the Axin protein complex, Glycogen Synthase Kinase-3 beta (GSK-3 beta) and Casein Kinase 1 (CK1) phosphorylate LRP-5/6 and the entire protein complex is subsequently internalized in endosomes that give rise to multivesicular bodies. Formation of multivesicular bodies containing the Wnt – Frizzled-LRP-5/6 – Axin protein complex sequesters GSK-3 beta from the cytosol and compromises its ability to phosphorylate newly synthesized beta-Catenin. Unphosphorylated beta-Catenin accumulates and translocates to the nucleus, where it associates with TCF/LEF family transcription factors and co-activators, such as Bcl-9 and Pygopus (Pygo), to induce the expression of Wnt target genes. In the absence of Wnt, cytoplasmic beta-Catenin is phosphorylated by CK1 and GSK-3 beta in the beta-Catenin destruction complex (Axin, APC, CK1, GSK-3 beta). These phosphorylation events create a docking site on beta-Catenin for beta-TrCP, an E3 ubiquitin ligase that promotes its ubiquitination and proteasomal degradation.
In addition to the beta-Catenin-dependent signaling pathway, there are two well-characterized beta-Catenin-independent Wnt signaling pathways, the PCP pathway and the Wnt/Ca2+ pathway. Both of these pathways inhibit Wnt-beta-Catenin-dependent signaling. The PCP pathway is initiated by Wnt binding to Frizzled receptors and a co-receptor such as ROR1/2, Ryk, or PTK7. Receptor binding leads to internalization of the ligand-receptor complex and downstream activation of the Rho and Rac GTPases, Rho-Kinase (ROCK), and c-Jun N-terminal kinase (JNK), which together regulate cell motility and tissue polarity. The Wnt-Ca2+ pathway is initiated by Frizzled receptors activating a classical G protein-coupled signaling pathway. Frizzled-G protein signaling activates Phospholipase C-beta (PLC-beta), which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). Production of DAG and IP3 results in the activation of Protein Kinase C (PKC), the Calcium/Calmodulin-dependent Protein Kinase type II (CaMKII)-TGF-beta-activated kinase 1 (TAK1)-Nemo-like kinase (NLK) pathway, and Calcineurin phosphatase, which dephosphorylates and activates the transcription factor, Nuclear Factor Associated with T cells (NFAT). Genes activated as a result of signaling through the Wnt/Ca2+ pathway regulate cell fate and cell migration.
Wnt proteins have also been shown to signal through a number of different co-receptors, including ROR2 and Ryk, which may or may not require Frizzled or another Wnt receptor. Wnt-5a-ROR2 signaling activates JNK to regulate convergent extension in Xenopus and may play a role in regulating the PCP pathway. In contrast, Wnt-Ryk signaling activates the beta-Catenin-dependent or PCP pathways and promotes axon guidance through Src activation. The PCP pathway has also been shown to be regulated during vertebrate development by MuSK and Vangl2. In muscle, MuSK functions as a Wnt receptor to organize a central muscle zone, while Vangl2 has been shown to form a receptor complex with either ROR2 or Ryk to mediate development of the neural tube, limbs, and cochlea. Deregulation of Wnt signaling is associated with a number of developmental disorders and human diseases including bone and renal diseases, type II diabetes, and cancer. Therefore, factors that regulate Wnt signaling are of great interest.
Wnt signaling is tightly regulated by multiple proteins that either enhance or inhibit Wnt activity. Wnt signaling through both the beta-Catenin-dependent pathway and the PCP pathway is enhanced by R-Spondins. R-Spondins bind to the leucine-rich repeat-containing, G protein-coupled receptors (Lgr) Lgr4, 5, or 6, and the transmembrane E3 ubiquitin ligase, RNF43/ZNRF3. In the absence of R-Spondins, RNF43/ZNRF3 ubiquitinates Frizzled receptors and promotes their internalization and subsequent degradation through the lysosomal pathway. Once bound by R-Spondins, the Lgr-R-Spondin-RNF43/ZNRF3 complex is internalized by endocytosis. Membrane clearance of RNF43/ZNRF3 stabilizes Wnt receptors and enhances Wnt responsiveness. Additionally, heparan sulphate proteoglycans (HSPGs) such as glypicans and syndecans enhance Wnt signaling by either increasing the amount of Wnt at the cell membrane or stabilizing the Wnt-Frizzled complex.
In addition to the positive regulators, Wnt signaling is also negatively regulated by several known antagonists that bind and inhibit either the Wnt protein itself or LRP-5/6. Wnt antagonists that interact directly with the Wnt protein include the secreted Frizzled-Related Proteins (sFRPs), Wnt Inhibitory Factor (WIF), Notum, and TIKI. Members of the sFRP family (sFRP-1-5) bind to Wnt proteins through their N-terminal cysteine-rich domains, which are homologous to the CRDs found in the Frizzled family of Wnt receptors. Binding of sFRP to Wnt prevents Wnt-receptor interactions and downstream signaling by both the beta-Catenin-dependent and PCP pathways. In addition, sFRPs can inhibit Wnt signaling by binding directly to Frizzled receptors. Similar to sFRPs, WIF is another secreted Wnt antagonist that prevents Wnt from binding to its receptor and thereby inhibits both the beta-Catenin dependent and PCP pathways. TIKI and Notum also inhibit Wnt signaling through these pathways, but unlike the sFRPs and WIF, these proteins are Wnt modifying enzymes that cleave the amino-terminal end of the Wnt protein or deacylate Wnt, respectively, reducing its receptor-binding capabilities. Wnt signaling can also be inhibited by LRP-5/6 antagonists including the Dkk proteins, Draxin, USAG1, SOST, and IGFBP-4. The Dkk family of secreted proteins functions as antagonists of beta-Catenin-dependent Wnt signaling by preventing the LRP-5/6 co-receptor from interacting with Wnt-Frizzled complexes, or by binding to Kremen-1 or -2, triggering LRP-5/6 internalization. Similarly, Draxin and the cysteine knot proteins, USAG1 and SOST, interact with LRP-5/6 and inhibit Wnt-induced Frizzled-LRP complex formation, while IGFBP-4 inhibits Wnt signaling by binding to both LRP-5/6 and Frizzled. TROY, a tumor necrosis factor receptor superfamily member has also recently been shown to inhibit Wnt signaling through an interaction with Lgr5.
To learn more, explore the following Wnt-related resources:
Wnt1 White Paper: Purification of Biologically Active sFRP/Wnt1 Complexes
Get Print Copy of this Pathway