ROR gamma t and Th17 Cells: New Highly-specific Antibody Will Find What You Have Been Missing
Antibody validation and specificity are not new headlines in the research community. Recent comparisons of R&D SystemsTM Human/Mouse ROR gamma t Recombinant Monoclonal Antibody (Clone # 1181A) to other ROR gamma t clones, demonstrate its superior performance. The data shown below suggest that a popular clone from another supplier is giving false negatives in mouse Th17-differentiated splenocytes and has markedly lower staining of ROR gamma t in human Th17 cells. R&D Systems strives to produce the highest quality antibodies through our recombinant monoclonal antibody generation or by only selecting the highest performing clones from our hybridoma-generated panels.
ROR gamma t is a commonly used marker in flow cytometry panels identifying Th17 cells. Naïve CD4+ T cells can differentiate into one of several T helper cell lineages depending on cytokines in the environment. In the presence of TGF beta and IL-6, CD4+ T cells can differentiate to Th17 cells, which are required for immune responses to certain extracellular bacteria and fungi. Th17 cells secrete cytokines IL-17A, IL-17F, and IL-22, all of which are driven by the master transcriptional regulator ROR gamma t. In addition to Th17 cells, ROR gamma t is expressed by various other lymphoid cell types, such as CD4+CD8+ thymocytes and CD8+ Th17 cells. Poor detection of ROR gamma t can hamper evaluation of cell populations that are secreting key Th17 cytokines.
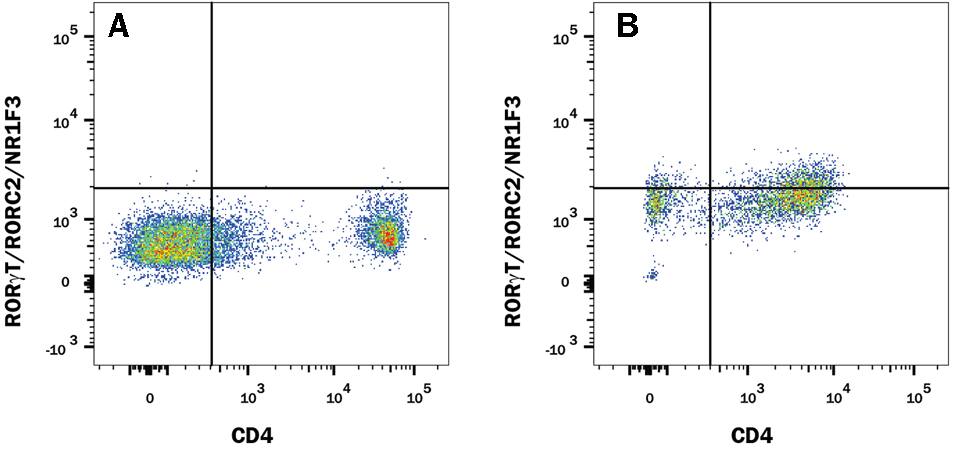
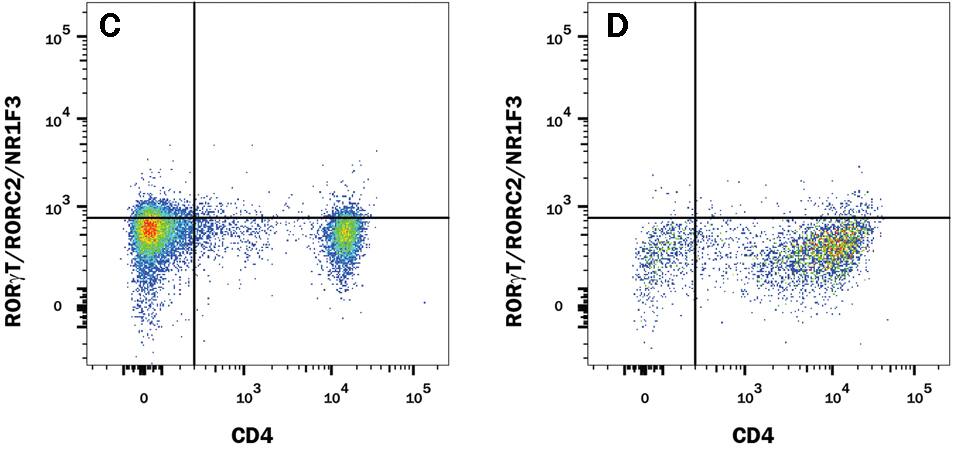
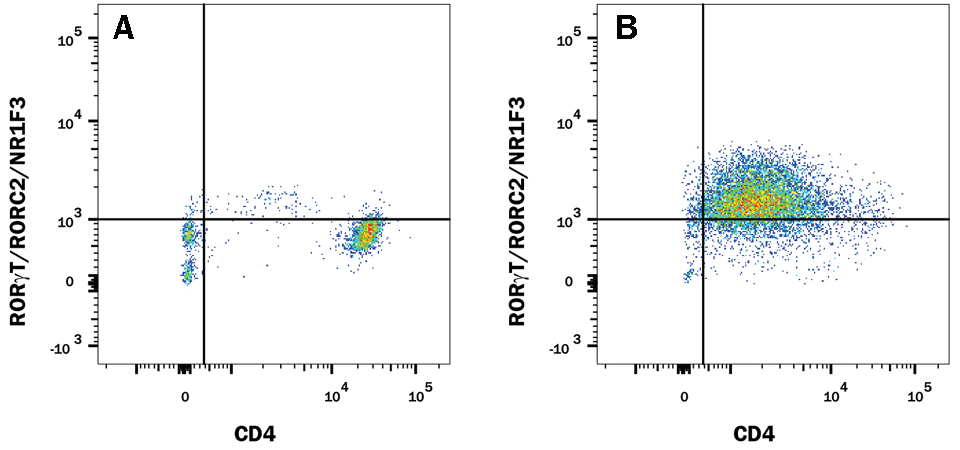
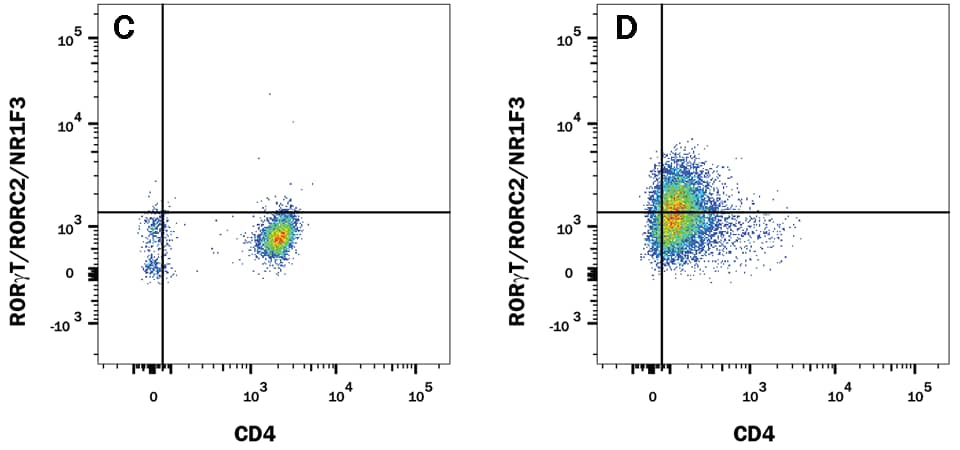
The research and development team at R&D Systems compared the performance of Human/Mouse ROR gamma t Recombinant Monoclonal Antibody (Clone # 1181A) versus an alternative supplier’s ROR gamma t clone. Expression was analyzed in resting and Th17-stimulated human peripheral blood mononuclear cells (PBMCs) as well as in resting and Th17-stimulated mouse splenocytes. The data shown below demonstrates a population of ROR gamma t+/CD4+ cell detected with clone # 1181A (plot B, both sample types) that is weakly detected or not detected by an alternative clone from a different antibody supplier (plot D, both sample types).
ROR gamma t staining in mouse Th17 cells
Detection of ROR gamma t/RORC2/NR1F3 in Mouse Splenocytes by Flow Cytometry. Mouse splenocytes either (A and C) unstimulated or (B and D) stimulated to induce Th17 cells were stained with an Alexa Fluor® 488-conjugated Rabbit Anti-Human/Mouse ROR gamma t/RORC2/NR1F3 Monoclonal Antibody (A and B, R&D Systems; Catalog # IC9125G) or C and D) Anti-Human/Mouse ROR gamma t (alternative clone from a different antibody supplier), and an APC-conjugated Rat Anti-Mouse CD4 Monoclonal Antibody (R&D Systems; Catalog # FAB554A). To facilitate intracellular staining, cells were fixed and permeabilized with FlowX™ FoxP3/Transcription Factor Fixation & Permeabilization Buffer Kit (R&D Systems; Catalog # FC012).
ROR gamma t staining in human Th17 cells
Detection of ROR gamma t/RORC2/NR1F3 in Human PBMCs by Flow Cytometry. Human PBMCs either (A and C) unstimulated or (B and D) stimulated to induce Th17 cells were stained with an Alexa Fluor® 488-conjugated Rabbit Anti-Human/Mouse ROR gamma t/RORC2/NR1F3 Monoclonal Antibody (A and B, R&D Systems; Catalog # IC9125G) or C and D) Anti-Human/Mouse ROR gamma t (alternative clone from a different antibody supplier), and APC-conjugated Mouse Anti-Human CD4 Monoclonal Antibody (R&D Systems; Catalog # FAB3791A). To facilitate intracellular staining, cells were fixed and permeabilized with FlowX™ FoxP3/Transcription Factor Fixation & Permeabilization Buffer Kit (R&D Systems; Catalog # FC012).
Get more information on other Th17 cell markers