Recombinant Human TNF-alpha, Animal-Free Protein
Recombinant Human TNF-alpha, Animal-Free Protein Summary
Learn more about Animal-Free Recombinant ProteinsAnimal Free Proteins
Animal-free proteins are particularly important for researchers concerned with experimental variables caused by trace animal components or mammalian pathogens. Our products generated under animal-free conditions share the same biological activities as those produced using our standard laboratory techniques.
Product Specifications
The specific activity of recombinant human TNF-alpha is >4.3 x 107 IU/mg, which is calibrated against the human TNF-alpha WHO International Standard (NIBSC code: 12/154).
Val77-Leu233, with and without an N-terminal Met
Produced using non-animal reagents in an animal-free laboratory.
Analysis
Product Datasheets
Carrier Free
CF stands for Carrier Free (CF). We typically add Bovine Serum Albumin (BSA) as a carrier protein to our recombinant proteins. Adding a carrier protein enhances protein stability, increases shelf-life, and allows the recombinant protein to be stored at a more dilute concentration. The carrier free version does not contain BSA.
In general, we advise purchasing the recombinant protein with BSA for use in cell or tissue culture, or as an ELISA standard. In contrast, the carrier free protein is recommended for applications, in which the presence of BSA could interfere.
AFL210
| Formulation | Lyophilized from a 0.2 μm filtered solution in PBS. |
| Reconstitution | Reconstitute at 0.2 mg/mL in sterile PBS. |
| Shipping | The product is shipped at ambient temperature. Upon receipt, store it immediately at the temperature recommended below. |
| Stability & Storage: | Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
|
Animal Free Proteins
Animal-free proteins are particularly important for researchers concerned with experimental variables caused by trace animal components or mammalian pathogens. Our products generated under animal-free conditions share the same biological activities as those produced using our standard laboratory techniques.
Reconstitution Calculator
Background: TNF-alpha
Tumor necrosis factor alpha (TNF-alpha ), also known as cachectin and TNFSF2, is the prototypic ligand of the TNF superfamily. It is a pleiotropic molecule that plays a central role in inflammation, immune system development, apoptosis, and lipid metabolism (1, 2). Human TNF-alpha consisits of a 35 amino acid (aa) cytoplasmic domain, a 21 aa transmembrane segment, and a 177 aa extracellular domain (ECD) (3). Within the ECD, human TNF-alpha shares 97% aa sequence identity with rhesus and 71%-92% with bovine, canine, cotton rat, equine, feline, mouse, porcine, and rat TNF-alpha. TNF-alpha is produced by a wide variety of immune, epithelial, endothelial, and tumor cells (1, 2). TNF-alpha is assembled intracellularly to form a noncovalently linked homotrimer which is expressed on the cell surface (4). Cell surface TNF-alpha can induce the lysis of neighboring tumor cells and virus infected cells, and it can generate its own downstream cell signaling following ligation by soluble TNFR I (2, 5). Shedding of membrane bound TNF-alpha by TACE/ADAM17 releases the bioactive cytokine, a 55 kDa soluble trimer of the TNF-alpha extracellular domain (6-8). TNF-alpha binds the ubiquitous 55-60 kDa TNF RI (9, 10) and the hematopoietic cell-restricted 80 kDa TNF RII (11, 12), both of which are also expressed as homotrimers (1, 2, 13). Both type I and type II receptors bind TNF-alpha with comparable affinity (14), although only TNF RI contains a cytoplasmic death domain which triggers the activation of apoptosis. Soluble forms of both types of receptors are released and can neutralize the biological activity of TNF-alpha (15).
- Zelova, H. and J. Hosek (2013) Inflamm. Res. 62:641.
- Juhasz, K. et al. (2013) Expert Rev. Clin. Immunol. 9:335.
- Pennica, D. et al. (1984) Nature 312:724.
- Tang, P. et al. (1996) Biochemistry 35:8216.
- Perez, C. et al. (1990) Cell 63:251.
- Black, R.A. et al. (1997) Nature 385:729.
- Moss, M.L. et al. (1997) Nature 385:733.
- Gearing, A.J.H. et al. (1994) Nature 370:555.
- Schall, T.J. et al. (1990) Cell 61:361.
- Loetscher, H. et al. (1990) Cell 61:351.
- Dembic, Z. et al. (1990) Cytokine 2:231.
- Smith, C.A. et al. (1990) Science 248:1019.
- Loetscher, H. et al. (1991) J. Biol. Chem. 266:18324.
- Pinckard, J.K. et al. (1997) J. Biol. Chem. 272:10784.
- Engelmann, H. et al. (1990) J. Biol. Chem. 265:1531.
Manufacturing Specifications
Animal-Free Manufacturing ConditionsOur dedicated controlled-access animal-free laboratories ensure that at no point in production are the products exposed to potential contamination by animal components or byproducts. Every stage of manufacturing is conducted in compliance with R&D Systems' stringent Standard Operating Procedures (SOPs). Production and purification procedures use equipment and media that are confirmed animal-free.
Production
- All molecular biology procedures use animal-free media and dedicated labware.
- Dedicated fermentors are utilized in committed animal-free areas.
Purification
- Protein purification columns are animal-free.
- Bulk proteins are filtered using animal-free filters.
- Purified proteins are stored in animal-free containers in a dedicated cold storage room.
- Low Endotoxin Level.
- No impairment of biological activity.
- High quality product obtained under stringent conditions.
- For ex vivo research or bioproduction, additional documentation can be provided.
FAQs
No product specific FAQs exist for this product, however you may
View all Proteins and Enzyme FAQsReviews for Recombinant Human TNF-alpha, Animal-Free Protein
Average Rating: 5 (Based on 1 Review)
Have you used Recombinant Human TNF-alpha, Animal-Free Protein?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
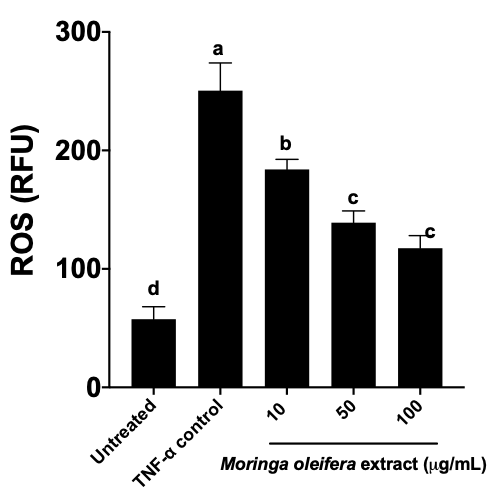
Reason for Rating: TNF-a protein was used successfully as a control of mice-derived pre-adipocytes activation during their maturation to adipocytes.
C57BL/6J-derived pre-adipocytes were extracted from adipose tissue. After culturing for 3 weeks, cells were stimulated with TNF-alpha as pro-inflammatory control, while the other cells were treated with a Moringa oleifera extract (10, 50, and 100 micrograms/mL). The quantification of Reactive oxygen species in the cells showed an adequate impact of TNF-alpha inducing inflammation and ROS activation. ROS were measured by fluorescence in relative fluorescence units (RFU).