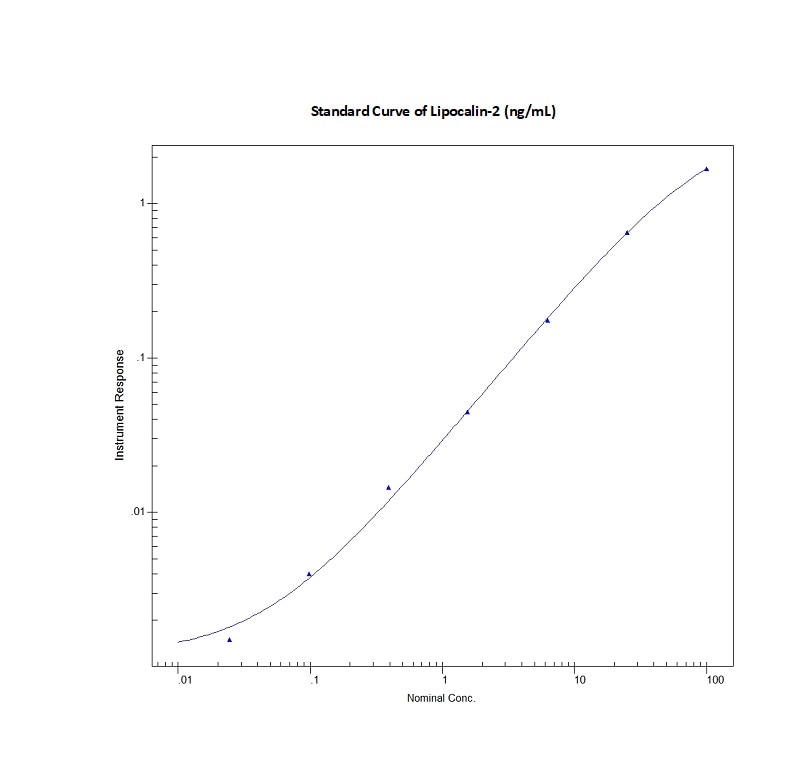
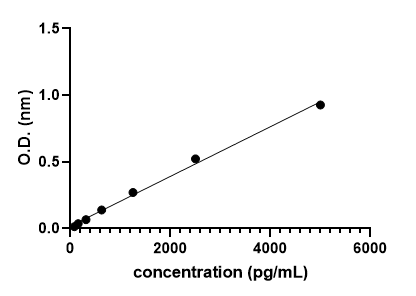
Mouse Lipocalin-2/NGAL Quantikine ELISA Kit Summary
Sample Values
Serum/Platelet-poor Plasma/Urine - Samples were evaluated for the presence of mouse Lipocalin-2 in this assay.| Sample Type | Mean (ng/mL) | Range (ng/mL) | Standard Deviation (ng/mL) |
| Serum (n=20) | 141 | 58.3-290 | 60.2 |
| Platelet-poor heparin plasma (n=20) | 76.0 | 40.7-143 | 30.1 |
| Platelet-poor EDTA plasma (n=20) | 54.1 | 32.5-74.7 | 12.3 |
| Urine (n=20) | 48.6 | 12.9-168 | 34.1 |
Cell Culture Supernates:
| Condition | Observed Values (ng/mL) |
| Unstimulated | 0.817 |
| Stimulated | 161 |
| Condition | Observed Values (ng/mL) |
| Unstimulated | 83.7 |
| Stimulated | 178 |
Two mouse brains and kidneys were homogenized and seeded in RPMI 1640 supplemented with 10% fetal bovine serum, 5 μM beta -mercaptoethanol, 2 mM L-glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin sulfate. The resulting supernates were either untreated or stimulated with 1 μg/mL of LPS for 3 days. Aliquots of the cell culture supernates were removed and assayed for levels of mouse Lipocalin-2.
| Condition | Observed Values (ng/mL) |
| Brain unstimulated | 0.334 |
| Brain stimulated | 178 |
| Condition | Observed Values (ng/mL) |
| Kidney Unstimulated | 1.28 |
| Kidney Stimulated | 4.69 |
Product Summary
Precision
Cell Culture Supernates, Serum, Platelet-poor EDTA Plasma, Platelet-poor Heparin Plasma, Urine
| Intra-Assay Precision | Inter-Assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 40 | 40 | 40 |
| Mean (pg/mL) | 196 | 476 | 1534 | 211 | 477 | 1564 |
| Standard Deviation | 13.2 | 19.6 | 65.9 | 15.2 | 28.6 | 98.5 |
| CV% | 6.7 | 4.1 | 4.3 | 7.2 | 6 | 6.3 |
Recovery
The recovery of mouse Lipocalin-2 spiked to three levels throughout the range of the assay was evaluated.
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Samples (n=4) | 93 | 82-101 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: Lipocalin-2/NGAL
The Lipocalin family comprises a diverse group of mostly secreted soluble proteins that bind hydrophobic ligands and act as transporters, carrying small molecules to specific cells. Lipocalins are related by possessing an 8-stranded beta-barrel structure. Lipocalin-1, also named tear lipocalin (TL), von Ebners gland protein (VEG) and tear pre-albumin, binds a large number of hydrophobic molecules and exhibits cysteine proteinase inhibitor and endonuclear activities. Lipocalin-2, also known as neutrophil gelatinase-associated lipocalin (NGAL), is a component of granules in neutrophils from tissues that are normally exposed to microorganisms and is upregulated during inflammation. Lipocalin-2 can form homodimers and can heterodimerize with the neutrophil gelatinase MMP-9.
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 50 µL of Assay Diluent to each well.
- Add 50 µL of Standard, Control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process 3 times for a total of 4 washes.
- Add 100 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 4 times.
- Add 100 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 100 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Mouse Lipocalin-2/NGAL Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
63
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Antidepressant Effects of Ginsenoside Rc on L-Alpha-Aminoadipic Acid-Induced Astrocytic Ablation and Neuroinflammation in Mice
Authors: Kwon, D;Kim, Y;Cho, SH;
International journal of molecular sciences
Species: Mouse
Sample Types: Tissue Homogenates
-
Serum and urinary biomarkers of vancomycin-induced acute kidney injury: A prospective, observational analysis
Authors: Park, SI;Yu, U;Oh, WS;Ryu, SW;Son, S;Lee, S;Baek, H;Park, JI;
Medicine
Species: Human
Sample Types: Urine
-
Activation of the osteoblastic HIF-1? pathway partially alleviates the symptoms of STZ-induced type 1 diabetes mellitus via RegIII?
Authors: Qiu, M;Chang, L;Tang, G;Ye, W;Xu, Y;Tulufu, N;Dan, Z;Qi, J;Deng, L;Li, C;
Experimental & molecular medicine
Species: Transgenic Mouse
Sample Types: Serum
-
Antidepressant Effect of Heracleum moellendorffii Extract on Behavioral Changes in Astrocyte Ablation Mouse Model of Depression by Modulating Neuroinflammation through the Inhibition of Lipocalin-2
Authors: Hong, S;Kim, Y;Kwon, Y;Cho, SH;
Nutrients
Species: Mouse
Sample Types: Tissue Homogenates
-
Lipocalin-2 as a prognostic marker in patients with acute exacerbation of idiopathic pulmonary fibrosis
Authors: Tanahashi, H;Iwamoto, H;Yamaguchi, K;Sakamoto, S;Horimasu, Y;Masuda, T;Nakashima, T;Ohshimo, S;Fujitaka, K;Hamada, H;Hattori, N;
Respiratory research
Species: Mouse
Sample Types: Serum
-
Mesenchymal Stem Cells Delivered Locally to Ischemia-Reperfused Kidneys via Injectable Hyaluronic Acid Hydrogels Decrease Extracellular Matrix Remodeling 1 Month after Injury in Male Mice
Authors: Han, DS;Erickson, C;Hansen, KC;Kirkbride-Romeo, L;He, Z;Rodell, CB;Soranno, DE;
Cells
Species: Mouse
Sample Types: Urine
-
IL-17C neutralization protects the kidney against acute injury and chronic injury
Authors: Zhang, F;Yin, J;Liu, L;Liu, S;Zhang, G;Kong, Y;Wang, Y;Wang, N;Chen, X;Wang, F;
EBioMedicine
Species: Mouse
Sample Types: Serum
-
T cell metabolic reprogramming in acute kidney injury and protection by glutamine blockade
Authors: Lee, K;Thompson, EA;Gharaie, S;Patel, CH;Kurzhagen, JT;Pierorazio, PM;Arend, LJ;Thomas, AG;Noel, S;Slusher, BS;Rabb, H;
JCI insight
Species: Mouse
Sample Types: Plasma
-
Soluble TNF mediates amyloid-independent, diet-induced alterations to immune and neuronal functions in an Alzheimer's disease mouse model
Authors: KP MacPherson, LN Eidson, MC Houser, BE Weiss, JL Gollihue, MK Herrick, ME de Sousa R, L Sniffen, EM Weekman, AM Hamilton, SD Kelly, DL Oliver, Y Yang, J Chang, TR Sampson, CM Norris, MG Tansey
Frontiers in Cellular Neuroscience, 2023-03-15;17(0):895017.
Species: Mouse
Sample Types: Plasma
-
Glutamine prevents acute kidney injury by modulating oxidative stress and apoptosis in tubular epithelial cells
Authors: K Thomas, L Zondler, N Ludwig, M Kardell, C Lüneburg, K Henke, S Mersmann, A Margraf, T Spieker, T Tekath, A Velic, R Holtmeier, J Hermann, V Jankowski, M Meersch, D Vestweber, M Westphal, J Roth, MA Schäfers, JA Kellum, CA Lowell, J Rossaint, A Zarbock
JCI Insight, 2022-11-08;7(21):.
Species: Mouse
Sample Types: Urine
-
Oral administration of human carbonic anhydrase I suppresses colitis in a murine inflammatory bowel disease model
Authors: K Tange, S Yagi, E Takeshita, M Abe, Y Yamamoto, H Tomida, T Kawamura, M Hanayama, B Matsuura, Y Ikeda, Y Hiasa
Scientific Reports, 2022-10-26;12(1):17983.
Species: Mouse
Sample Types: Feces
-
Immune-mediated tubule atrophy promotes acute kidney injury to chronic kidney disease transition
Authors: L Xu, J Guo, DG Moledina, LG Cantley
Nature Communications, 2022-08-19;13(1):4892.
Species: Mouse
Sample Types: Serum
-
Effects of Suramin on Polycystic Kidney Disease in a Mouse Model of Polycystin-1 Deficiency
Authors: MY Chang, SH Hsu, LY Ma, LF Chou, CC Hung, YC Tian, CW Yang
International Journal of Molecular Sciences, 2022-07-31;23(15):.
Species: Mouse
Sample Types: Plasma
-
Maternal heme-enriched diet promotes a gut pro-oxidative status associated with microbiota alteration, gut leakiness and glucose intolerance in mice offspring
Authors: A Mazenc, L Mervant, C Maslo, C Lencina, V Bézirard, M Levêque, I Ahn, V Alquier-Ba, N Naud, C Héliès-Tou, L Debrauwer, S Chevolleau, F Guéraud, FHF Pierre, V Théodorou, M Olier
Redox Biology, 2022-05-12;53(0):102333.
Species: Mouse
Sample Types: Feces
-
Role of lipocalin-2 in surgery-induced cognitive decline in mice: a signal from neuron to microglia
Authors: X Xiang, X Tang, Y Yu, S Xie, L Liu, M Chen, R Zhang, X Kang, Y Zheng, G Yang, S Gan, S Zhu
Journal of Neuroinflammation, 2022-04-12;19(1):92.
Species: Mouse
Sample Types: Serum
-
Snapshots of nascent RNA reveal cell- and stimulus- specific responses to acute kidney injury
Authors: TH Shen, J Stauber, K Xu, A Jacunski, N Paragas, M Callahan, R Banlengchi, AD Levitman, B Desanti de, A Beenken, MS Grau, E Mathieu, Q Zhang, Y Li, T Gopal, N Askanase, S Arumugam, S Mohan, PI Good, JS Stevens, F Lin, SK Sia, CS Lin, V D'Agati, K Kiryluk, NP Tatonetti, J Barasch
JCI Insight, 2022-03-22;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oligosaccharides Ameliorate Acute Kidney Injury by Alleviating Cluster of Differentiation 44-Mediated Immune Responses in Renal Tubular Cells
Authors: TH Chen, CT Liu, CY Cheng, YM Sue, NJ Huang, CH Chen
Nutrients, 2022-02-11;14(4):.
Species: Rat
Sample Types: Serum
-
Resident Self-Tissue of Proinflammatory Cytokines Rather Than Their Systemic Levels Correlates with Development of Myelofibrosis in Gata1low Mice
Authors: M Zingariell, P Verachi, F Gobbo, F Martelli, M Falchi, M Mazzarini, M Valeri, G Sarli, C Marinaccio, J Melo-Carde, JD Crispino, AR Migliaccio
Biomolecules, 2022-01-30;12(2):.
Species: Mouse
Sample Types: Serum
-
MicroRNA?93 inhibits the apoptosis and inflammatory response of tubular epithelial cells via the PTEN/AKT/mTOR pathway in acute kidney injury
Authors: Y Zhan, M Zhu, S Liu, J Lu, Z Ni, H Cai, W Zhang
Molecular Medicine Reports, 2021-07-23;24(3):.
Species: Mouse
Sample Types: Serum
-
Gut microbiome-host interactions in driving environmental pollutant trichloroethene-mediated autoimmunity
Authors: H Wang, N Banerjee, Y Liang, G Wang, KL Hoffman, MF Khan
Toxicology and Applied Pharmacology, 2021-05-27;424(0):115597.
Species: Mouse
Sample Types: Serum
-
Angiotensin AT2 Receptor is Anti-inflammatory and Reno-Protective in Lipopolysaccharide Mice Model: Role of IL-10
Authors: N Fatima, S Patel, T Hussain
Frontiers in Pharmacology, 2021-04-15;12(0):600163.
Species: Mouse
Sample Types: Tissue Homogenates
-
Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon
Authors: A Nakamura, S Kurihara, D Takahashi, W Ohashi, Y Nakamura, S Kimura, M Onuki, A Kume, Y Sasazawa, Y Furusawa, Y Obata, S Fukuda, S Saiki, M Matsumoto, K Hase
Nature Communications, 2021-04-08;12(1):2105.
Species: Mouse
Sample Types: Faecal supernates
-
Effects of selective TNFR1 inhibition or TNFR2 stimulation, compared to non-selective TNF inhibition, on (neuro)inflammation and behavior after myocardial infarction in male mice
Authors: L Gouweleeuw, H Wajant, O Maier, ULM Eisel, WM Blankestei, RG Schoemaker
Brain, Behavior, and Immunity, 2021-01-12;0(0):.
Species: Mouse
Sample Types: Plasma
-
Conditional Myh9 and Myh10 inactivation in adult mouse renal epithelium results in progressive kidney disease
Authors: KL Otterpohl, BW Busselman, I Ratnayake, RG Hart, K Hart, C Evans, CL Phillips, JR Beach, P Ahrenkiel, B Molitoris, K Surendran, I Chandrasek
JCI Insight, 2020-11-05;0(0):.
Species: Mouse
Sample Types: Serum
-
LPS-Induced Acute Kidney Injury Is Mediated by Nox4-SH3YL1
Authors: JY Yoo, DR Cha, B Kim, EJ An, SR Lee, JJ Cha, YS Kang, JY Ghee, JY Han, YS Bae
Cell Rep, 2020-10-20;33(3):108245.
Species: Mouse
Sample Types: Serum
-
Anti-Inflammatory and Gut Microbiota Modulatory Effect of Lactobacillus rhamnosus Strain LDTM 7511 in a Dextran Sulfate Sodium-Induced Colitis Murine Model
Authors: S Yeo, H Park, E Seo, J Kim, BK Kim, IS Choi, CS Huh
Microorganisms, 2020-06-04;8(6):.
Species: Mouse
Sample Types: Fecal Supernates
-
Sodium-glucose cotransporter 2 inhibition suppresses HIF-1&alpha-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice
Authors: T Cai, Q Ke, Y Fang, P Wen, H Chen, Q Yuan, J Luo, Y Zhang, Q Sun, Y Lv, K Zen, L Jiang, Y Zhou, J Yang
Cell Death Dis, 2020-05-22;11(5):390.
Species: Human
Sample Types: Urine
-
Intravenous Arginine Administration Downregulates NLRP3 Inflammasome Activity and Attenuates Acute Kidney Injury in Mice with Polymicrobial Sepsis
Authors: SA Tanusepute, MT Lin, SL Yeh, CL Yeh
Mediators Inflamm., 2020-05-11;2020(0):3201635.
Species: Mouse
Sample Types: Plasma
-
Lipocalin2 Induced by Bacterial Flagellin Protects Mice against Cyclophosphamide Mediated Neutropenic Sepsis
Authors: D Lim, HK Kim, JH Jeong, YS Jung, SE Lee, HC Jang, SI Jung, HS Choi, JH Rhee, SG Lee, C Park, M Song, HE Choy
Microorganisms, 2020-04-29;8(5):.
Species: Mouse
Sample Types: Serum
-
A Novel Approach to Deliver Therapeutic Extracellular Vesicles Directly into the Mouse Kidney via Its Arterial Blood Supply
Authors: M Ullah, DD Liu, S Rai, M Razavi, J Choi, J Wang, W Concepcion, AS Thakor
Cells, 2020-04-10;9(4):.
Species: Mouse
Sample Types: Serum
-
CXCL14 Overexpression Attenuates Sepsis-Associated Acute Kidney Injury by Inhibiting Proinflammatory Cytokine Production
Authors: J Lv, ZL Wu, Z Gan, P Gui, SL Yao
Mediators Inflamm., 2020-03-31;2020(0):2431705.
Species: Mouse
Sample Types: Tissue Homogenate
-
Targeting soluble tumor necrosis factor as a potential intervention to lower risk for late-onset Alzheimer's disease associated with obesity, metabolic syndrome, and type 2 diabetes
Authors: ME De Sousa R, MC Houser, DI Walker, DP Jones, J Chang, CJ Barnum, MG Tansey
Alzheimers Res Ther, 2019-12-31;12(1):1.
Species: Mouse
Sample Types: Plasma
-
Lipocalin-2 Regulates Epidermal Growth Factor Receptor Intracellular Trafficking
Authors: L Yammine, A Zablocki, W Baron, F Terzi, M Gallazzini
Cell Rep, 2019-11-12;29(7):2067-2077.e6.
Species: Mouse
Sample Types: cell culture supernatant
-
Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease
Authors: EC Lee, T Valencia, C Allerson, A Schairer, A Flaten, M Yheskel, K Kersjes, J Li, S Gatto, M Takhar, S Lockton, A Pavlicek, M Kim, T Chu, R Soriano, S Davis, JR Androsavic, S Sarwary, T Owen, J Kaplan, K Liu, G Jang, S Neben, P Bentley, T Wright, V Patel
Nat Commun, 2019-09-12;10(1):4148.
Species: Mouse
Sample Types: Urine
-
Lipocalin-2 (Lcn-2) Attenuates Polymicrobial Sepsis with LPS Preconditioning (LPS Tolerance) in FcGRIIb Deficient Lupus Mice
Authors: T Ondee, J Gillen, P Visitchana, P Somparn, J Issara-Amp, C Dang Phi, W Chancharoe, D Gurusamy, A Nita-Lazar, A Leelahavan
Cells, 2019-09-11;8(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Modulation of the lung inflammatory response to ozone by the estrous cycle
Authors: N Fuentes, N Cabello, M Nicoleau, ZC Chroneos, P Silveyra
Physiol Rep, 2019-03-01;7(5):e14026.
Species: Mouse
Sample Types: BALF
-
Negr1 controls adult hippocampal neurogenesis and affective behaviors
Authors: K Noh, H Lee, TY Choi, Y Joo, SJ Kim, H Kim, JY Kim, JW Jahng, S Lee, SY Choi, SJ Lee
Mol. Psychiatry, 2019-01-16;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions
Authors: N Makhezer, M Ben Khemis, D Liu, Y Khichane, V Marzaioli, A Tlili, M Mojallali, C Pintard, P Letteron, M Hurtado-Ne, J El-Benna, JC Marie, A Sannier, AL Pelletier, PM Dang
Mucosal Immunol, 2018-10-02;0(0):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer
Authors: J Steenbrugg, K Breyne, K Demeyere, O De Wever, NN Sanders, W Van Den Br, C Colpaert, P Vermeulen, S Van Laere, E Meyer
J. Exp. Clin. Cancer Res., 2018-08-15;37(1):191.
Species: Mouse
Sample Types: Serum
-
Effects of lobeglitazone on insulin resistance and hepatic steatosis in high-fat diet-fed mice
Authors: BH Choi, Z Jin, CO Yi, J Oh, EA Jeong, JY Lee, KA Park, KE Kim, JE Lee, HJ Kim, JR Hahm, GS Roh
PLoS ONE, 2018-07-06;13(7):e0200336.
Species: Mouse
Sample Types: Serum
-
RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction
Authors: A Sureshbabu, E Patino, KC Ma, K Laursen, EJ Finkelszte, O Akchurin, T Muthukumar, SW Ryter, L Gudas, AMK Choi, ME Choi
JCI Insight, 2018-06-07;3(11):.
Species: Mouse
Sample Types: Urine
-
Endothelium-targeted delivery of dexamethasone by anti-VCAM-1 SAINT-O-Somes in mouse endotoxemia
Authors: R Li, PS Kowalski, HWM Morselt, I Schepel, RM Jongman, A Aslan, MHJ Ruiters, JG Zijlstra, G Molema, M van Meurs, JAAM Kamps
PLoS ONE, 2018-05-15;13(5):e0196976.
Species: Mouse
Sample Types: Plasma
-
RNA Nanotherapeutics for the Amelioration of Astroglial Reactivity
Authors: JA Smith, A Braga, J Verheyen, S Basilico, S Bandiera, C Alfaro-Cer, L Peruzzotti, D Shu, F Haque, P Guo, S Pluchino
Mol Ther Nucleic Acids, 2017-11-24;10(0):103-121.
Species: Mouse
Sample Types: Whole Cells
Applications: ELISA Capture -
Ameliorative Effect of Daidzein on Cisplatin-Induced Nephrotoxicity in Mice via Modulation of Inflammation, Oxidative Stress, and Cell Death
Authors: H Meng, G Fu, J Shen, K Shen, Z Xu, Y Wang, B Jin, H Pan
Oxid Med Cell Longev, 2017-08-02;2017(0):3140680.
Species: Mouse
Sample Types: Serum
-
CD40-signalling abrogates induction of ROR?t(+) Treg cells by intestinal CD103(+) DCs and causes fatal colitis
Authors: C Barthels, A Ogrinc, V Steyer, S Meier, F Simon, M Wimmer, A Blutke, T Straub, U Zimber-Str, E Lutgens, P Marconi, C Ohnmacht, D Garzetti, B Stecher, T Brocker
Nat Commun, 2017-03-09;8(0):14715.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment
Authors: S Gomez-Chou, A Swidnicka-, N Badi, M Chavez-Tom, GB Lesinski, T Bekaii-Saa, MR Farren, TA Mace, C Schmidt, Y Liu, D Deng, RF Hwang, L Zhou, TT Moore, D Chatterjee, H Wang, X Leng, RB Arlinghaus, CD Logsdon, Z Cruz-Monse
Cancer Res, 2017-03-01;0(0):.
Species: Mouse
Sample Types: Serum
-
Autophagy Inhibits the Accumulation of Advanced Glycation End Products by Promoting Lysosomal Biogenesis and Function in the Kidney Proximal Tubules
Authors: A Takahashi, Y Takabatake, T Kimura, I Maejima, T Namba, T Yamamoto, J Matsuda, S Minami, JY Kaimori, I Matsui, T Matsusaka, F Niimura, T Yoshimori, Y Isaka
Diabetes, 2017-02-28;0(0):.
Species: Mouse
Sample Types: Urine
-
Human Alpha-1-Antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia-reperfusion injury
Authors: N Maicas, J van der Vl, J Bublitz, S Florquin, M Bakker-van, CA Dinarello, V Verweij, R Masereeuw, LA Joosten, LB Hilbrands
PLoS ONE, 2017-02-24;12(2):e0168981.
Species: Mouse
Sample Types: Plasma
-
The effects of exogenous desmopressin on a model of heat stress nephropathy in mice
Authors: Carlos A Roncal-Jim
Am. J. Physiol. Renal Physiol, 2016-12-21;0(0):ajprenal.0049.
Species: Mouse
Sample Types: Urine
-
Obesity-induced kidney injury is attenuated by amelioration of aberrant PHD2 activation in proximal tubules
Sci Rep, 2016-11-09;6(0):36533.
Species: Mouse
Sample Types: Urine
-
Chronic Repression of mTOR Complex 2 Induces Changes in the Gut Microbiota of Diet-induced Obese Mice
Sci Rep, 2016-07-29;6(0):30887.
Species: Mouse
Sample Types: Feces
-
Acute Stress Responses Are Early Molecular Events of Retinal Degeneration in Abca4-/-Rdh8-/- Mice After Light Exposure
Authors: Tanu Parmar
Invest Ophthalmol Vis Sci, 2016-06-01;57(7):3257-67.
Species: Mouse, Transgenic Mouse
Sample Types: Tissue Homogenates
-
Renal Impairment with Sublethal Tubular Cell Injury in a Chronic Liver Disease Mouse Model.
Authors: Ishida T, Kotani H, Miyao M, Kawai C, Jemail L, Abiru H, Tamaki K
PLoS ONE, 2016-01-11;11(1):e0146871.
Species: Mouse
Sample Types: Urine
-
Exogenous Lipocalin 2 Ameliorates Acute Rejection in a Mouse Model of Renal Transplantation
Authors: F Aigner
Am. J. Transplant., 2015-11-23;16(3):808-20.
Species: Mouse
Sample Types: Serum
-
Lipocalin-2 promotes m1 macrophages polarization in a mouse cardiac ischaemia-reperfusion injury model.
Authors: Cheng L, Xing H, Mao X, Li L, Li X, Li Q
Scand J Immunol, 2015-01-01;81(1):31-8.
Species: Mouse
Sample Types: Serum
-
Lipocalin2 as a plasma marker for tumors with hypoxic regions.
Authors: Nakamura I, Hama S, Itakura S, Takasaki I, Nishi T, Tabuchi Y, Kogure K
Sci Rep, 2014-12-03;4(0):7235.
Species: Mouse
Sample Types: Plasma
-
Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2.
Authors: Miao Q, Ku A, Nishino Y, Howard J, Rao A, Shaver T, Garcia G, Le D, Karlin K, Westbrook T, Poli V, Nguyen H
Nat Commun, 2014-06-09;5(0):4088.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ghrelin protects against renal damages induced by angiotensin-II via an antioxidative stress mechanism in mice.
Authors: Fujimura K, Wakino S, Minakuchi H, Hasegawa K, Hosoya K, Komatsu M, Kaneko Y, Shinozuka K, Washida N, Kanda T, Tokuyama H, Hayashi K, Itoh H
PLoS ONE, 2014-04-18;9(4):e94373.
Species: Mouse
Sample Types: Urine
-
The small fibrinopeptide Bbeta15-42 as renoprotective agent preserving the endothelial and vascular integrity in early ischemia reperfusion injury in the mouse kidney.
Authors: Urbschat A, Zacharowski K, Obermuller N, Rupprecht K, Penzkofer D, Jennewein C, Tran N, Scheller B, Dimmeler S, Paulus P
PLoS ONE, 2014-01-02;9(1):e84432.
Species: Mouse
Sample Types: Serum
-
Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-alpha antibodies.
Authors: Bhargava R, Altmann C, Andres-Hernando A, Webb R, Okamura K, Yang Y, Falk S, Schmidt E, Faubel S
PLoS ONE, 2013-11-12;8(11):e79037.
Species: Mouse
Sample Types: Urine
-
ATP release and autocrine signaling through P2X4 receptors regulate gammadelta T cell activation.
Authors: Manohar M, Hirsh M, Chen Y, Woehrle T, Karande A, Junger W
J Leukoc Biol, 2012-06-29;92(4):787-94.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus.
Authors: Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C
Infect. Immun., 2011-10-28;80(1):410-7.
Species: Mouse
Sample Types: Tissue Homogenates
-
Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2.
Authors: Nairz M, Theurl I, Schroll A, Theurl M, Fritsche G, Lindner E, Seifert M, Crouch ML, Hantke K, Akira S, Fang FC, Weiss G
Blood, 2009-08-21;114(17):3642-51.
Species: Mouse
Sample Types: Serum
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse Lipocalin-2/NGAL Quantikine ELISA Kit
Average Rating: 4.8 (Based on 5 Reviews)
Have you used Mouse Lipocalin-2/NGAL Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
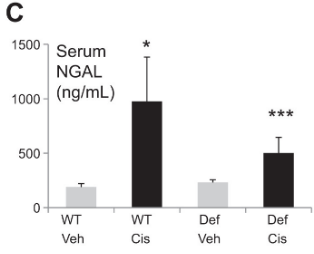
Wild type (WT) or IL33-/- (Def) mice were given either vehicle (Veh) or cisplatin (Cis) to induce kidney injury. Serum NGAL was measured as a marker of kidney injury.
Ravichandran et. al. AJP Renal 2017
Almost as good as human kit.