Mouse CD3 Antibody Summary
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
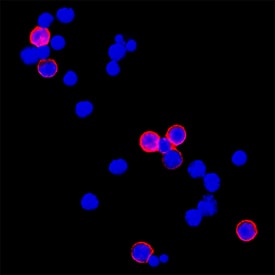
CD3 in Mouse Splenocytes. CD3 was detected in immersion fixed mouse splenocytes using Rat Anti-Mouse CD3 Monoclonal Antibody (Catalog # MAB4841) at 3 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (red; NL013) and counterstained with DAPI (blue). Specific staining was localized to plasma membrane. View our protocol for Fluorescent ICC Staining of Non-adherent Cells.
 View Larger
View Larger
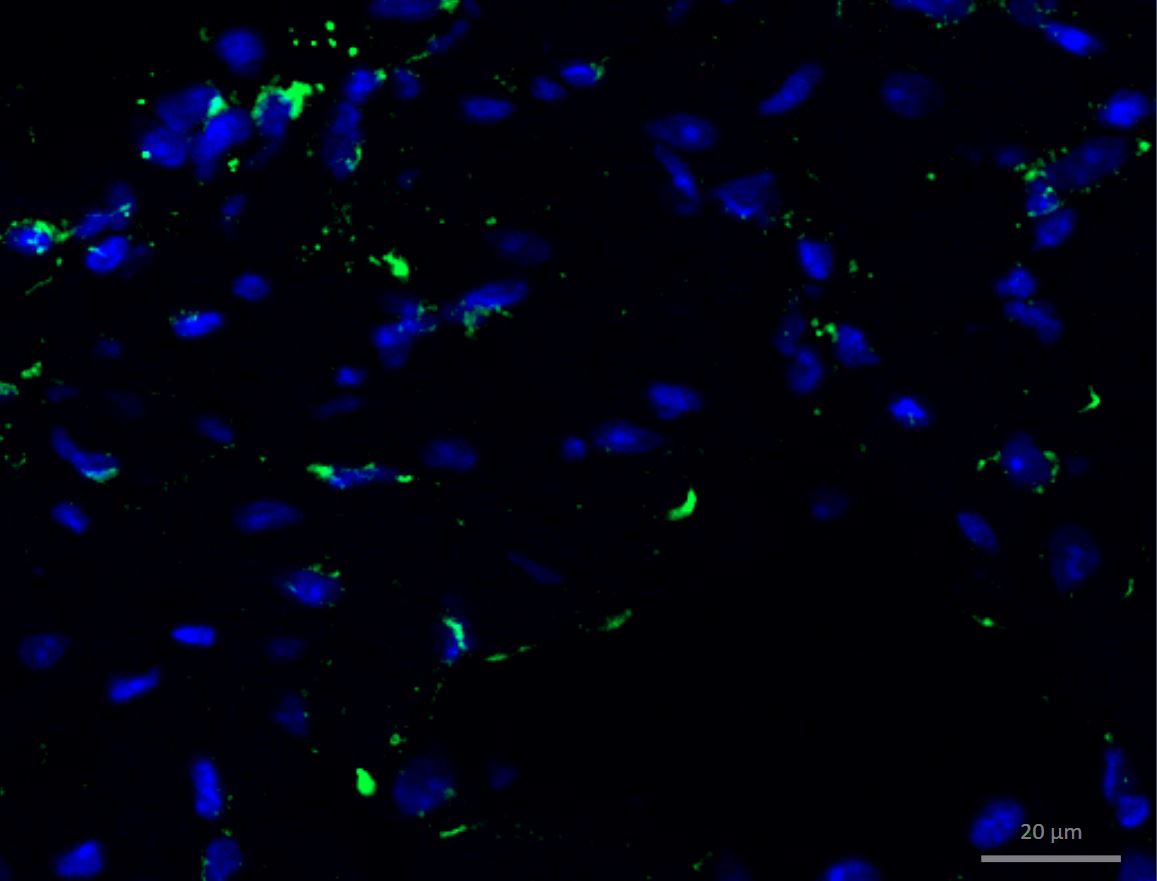
CD3 in Mouse Brain. CD3 was detected in perfusion fixed HSV-1 infected frozen sections of mouse brain (perivascular cuffing) using 10 µg/mL Rat Anti-Mouse CD3 Monoclonal Antibody (Catalog # MAB4841) overnight at 4 °C. Tissue was stained using the NorthernLights™ 493-conjugated Anti-Rat IgG Secondary Antibody (green; NL015) and counterstained with DAPI (blue). View our protocol for Fluorescent IHC Staining of Frozen Tissue Sections.
 View Larger
View Larger
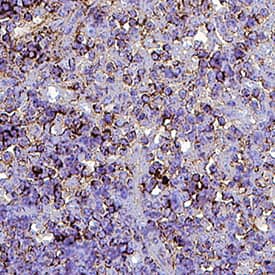
Detection of CD3 in Mouse Thymus. CD3 was detected in perfusion fixed paraffin-embedded sections of Mouse Thymus using Rat Anti-Mouse CD3 Monoclonal Antibody (Catalog # MAB4841) at 15 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Rat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC005). Before incubation with the primary antibody, tissue was subjected to heat-induced epitope retrieval using VisUCyte Antigen Retrieval Reagent-Basic (Catalog # VCTS021). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell surface of lymphocytes. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
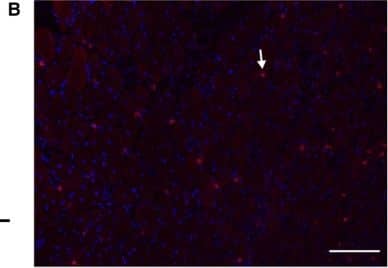
Detection of Mouse CD3 by Immunocytochemistry/Immunofluorescence (A) T cells (CD3+ cells) were increased at 2 and 7 days postinjury (DPI) compared to 14 DPI (*, P < 0.05) in injured muscles of both wild‐type (WT) and CXCL10 knockout (KO) mice. (B) A representative micrograph of a T‐cell stain (Red = CD3, Blue = DNA) in a WT mouse at 7 DPI. Arrows point out examples of CD3‐positive cells. (C) CXCR3‐positive cells were increased at 2 and 7 DPI compared to 14 DPI (*P < 0.05) in both genotypes. (D) A representative micrograph of CXCR3 (green) stain in a WT mouse at 7 DPI. Arrows mark examples of CXCR3‐positive cells. (E) Approximately 50% of the T cells (CD3 cells) were CXCR3 positive (the CXCL10 receptor). No differences were observed between genotypes or between 2 and 7 DPI. (F) A merged image of panel B and D. The arrow points out an example a cell positive for both CD3 and CXCR3. The circle shows a CD3‐positive, CXCR3‐negative cell. Data are mean ± SD. Scale bar is 100 μm. n = 4–6 per experimental condition. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29696819), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse CD3 by Immunocytochemistry/Immunofluorescence (A) T cells (CD3+ cells) were increased at 2 and 7 days postinjury (DPI) compared to 14 DPI (*, P < 0.05) in injured muscles of both wild‐type (WT) and CXCL10 knockout (KO) mice. (B) A representative micrograph of a T‐cell stain (Red = CD3, Blue = DNA) in a WT mouse at 7 DPI. Arrows point out examples of CD3‐positive cells. (C) CXCR3‐positive cells were increased at 2 and 7 DPI compared to 14 DPI (*P < 0.05) in both genotypes. (D) A representative micrograph of CXCR3 (green) stain in a WT mouse at 7 DPI. Arrows mark examples of CXCR3‐positive cells. (E) Approximately 50% of the T cells (CD3 cells) were CXCR3 positive (the CXCL10 receptor). No differences were observed between genotypes or between 2 and 7 DPI. (F) A merged image of panel B and D. The arrow points out an example a cell positive for both CD3 and CXCR3. The circle shows a CD3‐positive, CXCR3‐negative cell. Data are mean ± SD. Scale bar is 100 μm. n = 4–6 per experimental condition. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/29696819), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
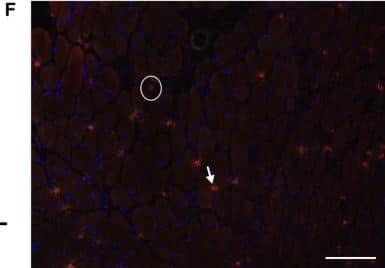
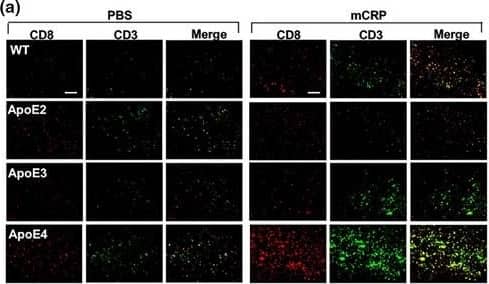
Detection of Mouse CD3 by Immunohistochemistry Peripheral mCRP‐induced neuroinflammation, extravasation of T lymphocytes&AD pathology traits in the ApoE4 brain. (a) We then investigated if mCRP‐induced cerebrovascular damage can migrate peripheral immune cells into the brain. Double immunostaining of CD8 (red), CD3 (green),&double‐positive cells (yellow) was performed to study the transcytosis of T lymphocytes in the cortex after i.p. treatment with PBS (left columns) versus mCRP (right columns) in WT&different ApoE knock‐in mice. Quantification of CD8+ T lymphocytes&CD8+/CD3+ T lymphocytes in the cortex&the comparisons between PBS versus mCRP treatment in each genotype was conducted. mCRP significantly increased the number of T lymphocytes only in the WT (p = 0.05)&ApoE4 (p = 0.001) mice. n = 7–8 in each condition. Image collected & cropped by CiteAb from the following open publication (https://pubmed.ncbi.nlm.nih.gov/34687487), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: CD3
Monoclonal 17A2 was generated by immunizing rats with a T-cell hybridoma (1). This antibody has been shown to react with the epsilon chain of the CD3 complex (1). The CD3 protein complex is expressed on thymocytes and mature T-cells and, therefore, is a useful reagent to monitor T-cell frequencies in tissues.
Product Datasheets
Citations for Mouse CD3 Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
42
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
T cells promote microglia-mediated synaptic elimination and cognitive dysfunction during recovery from neuropathogenic flaviviruses.
Authors: Garber C, Soung A et al.
Nat Neurosci
-
Eggerthella lenta augments preclinical autoantibody production and metabolic shift mimicking senescence in arthritis
Authors: Balakrishnan, B;Luckey, D;Wright, K;Davis, JM;Chen, J;Taneja, V;
Science advances
Species: Transgenic Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
ITK signaling regulates a switch between T helper 17 and T regulatory cell lineages via a calcium-mediated pathway
Authors: O Anannya, W Huang, A August
bioRxiv : the preprint server for biology, 2023-04-03;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Dll4 Inhibition Promotes Graft Retention in Fat Grafting Enriched with Adipose-Derived Stem Cells
Authors: Choong-kun Lee, Bo-Yoon Park, Taehee Jo, Cheol-Heum Park, Ju-Hee Kim, Kyu-Jin Chung et al.
Stem Cells Translational Medicine
-
Lack of ApoA-I in ApoEKO Mice Causes Skin Xanthomas, Worsening of Inflammation, and Increased Coronary Atherosclerosis in the Absence of Hyperlipidemia
Authors: Marco Busnelli, Stefano Manzini, Alice Colombo, Elsa Franchi, Fabrizia Bonacina, Matteo Chiara et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Ephrin receptor A10 monoclonal antibodies and the derived chimeric antigen receptor T cells exert an antitumor response in mouse models of triple-negative breast cancer
Authors: JH Cha, LC Chan, YN Wang, YY Chu, CH Wang, HH Lee, W Xia, WC Shyu, SP Liu, J Yao, CW Chang, FR Cheng, J Liu, SO Lim, JL Hsu, WH Yang, GN Hortobagyi, C Lin, L Yang, D Yu, LB Jeng, MC Hung
The Journal of Biological Chemistry, 2022-03-10;0(0):101817.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Generation of functional human thymic cells from induced pluripotent stem cells
Authors: Stephan A. Ramos, John J. Morton, Prabha Yadav, Brendan Reed, Sheila I. Alizadeh, Ali H. Shilleh et al.
Journal of Allergy and Clinical Immunology
-
PD-L1 (Programmed Death Ligand 1) Regulates T-Cell Differentiation to Control Adaptive Venous Remodeling
Authors: Yutaka Matsubara (松原 裕), Luis Gonzalez, Gathe Kiwan, Jia Liu (刘 佳), John Langford, Mingjie Gao (高 明杰) et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Monomeric C-reactive protein via endothelial CD31 for neurovascular inflammation in an ApoE genotype-dependent pattern: A risk factor for Alzheimer's disease?
Authors: Z Zhang, H Na, Q Gan, Q Tao, Y Alekseyev, J Hu, Z Yan, JB Yang, H Tian, S Zhu, Q Li, IM Rajab, JK Blusztajn, B Wolozin, A Emili, X Zhang, T Stein, LA Potempa, WQ Qiu
Aging Cell, 2021-10-23;0(0):e13501.
Species: Mouse
Sample Types: Whole Tissue
Applications: IF -
The YAP/HIF-1&alpha/miR-182/EGR2 axis is implicated in asthma severity through the control of Th17 cell differentiation
Authors: J Zhou, N Zhang, W Zhang, C Lu, F Xu
Cell & bioscience, 2021-05-12;11(1):84.
Species: Mouse
Sample Types: Whole Cells
Applications: Neutralization -
Itgb3-integrin-deficient mice may not be a sufficient�model for patients with Glanzmann thrombasthenia
Authors: D Li, J Peng, T Li, Y Liu, M Chen, X Shi
Molecular Medicine Reports, 2021-04-21;23(6):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Inhibition of T-Cells by Cyclosporine A Reduces Macrophage Accumulation to Regulate Venous Adaptive Remodeling and Increase Arteriovenous Fistula Maturation
Authors: Yutaka Matsubara (松原裕), Gathe Kiwan, Jia Liu (刘佳), Luis Gonzalez, John Langford, Mingjie Gao (高明杰) et al.
Arteriosclerosis, Thrombosis, and Vascular Biology
-
Hepatocyte cannabinoid 1 receptor nullification alleviates toxin-induced liver damage via NF-&kappaB signaling
Authors: Y Kim, S Gautam, KR Aseer, J Kim, P Chandrasek, CH Mazucanti, P Ghosh, JF O'Connell, ME Doyle, A Appleton, E Lehrmann, QR Liu, JM Egan
Cell Death & Disease, 2020-12-09;11(12):1044.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Cnp Promoter-Driven Sustained ERK1/2 Activation Increases B-Cell Activation and Suppresses Experimental Autoimmune Encephalomyelitis
Authors: Marisa A. Jeffries, Alison E. Obr, Kelly Urbanek, Sharyl L. Fyffe-Maricich, Teresa L. Wood
ASN Neuro
-
Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis
Authors: C Alvarez, S Suliman, R Almarhoumi, ME Vega, C Rojas, G Monasterio, M Galindo, R Vernal, A Kantarci
Sci Rep, 2020-11-04;10(1):19018.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Modeling human pediatric and adult gliomas in immunocompetent mice through costimulatory blockade
Authors: Xiaoyan Lan, Dorota A. Kedziorek, Chengyan Chu, Anna Jablonska, Shen Li, Mihoko Kai et al.
OncoImmunology
-
Prion protein signaling induces M2 macrophage polarization and protects from lethal influenza infection in mice
Authors: J Chida, H Hara, K Uchiyama, E Takahashi, H Miyata, H Kosako, Y Tomioka, T Ito, H Horiuchi, H Matsuda, H Kido, S Sakaguchi
PLoS Pathog., 2020-08-26;16(8):e1008823.
Species: Mouse
Sample Types: In Vivo, Whole Cells, Whole Tissue
Applications: Flow Cytometry, ICC, IHC, Neutralization -
Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy
Authors: YP Xu, L Lv, Y Liu, MD Smith, WC Li, XM Tan, M Cheng, Z Li, M Bovino, J Aubé, Y Xiong
J. Clin. Invest., 2019-07-16;130(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Contributions of T cell dysfunction to the resistance against anti-PD-1 therapy in oral carcinogenesis
Authors: L Wen, H Lu, Q Li, Q Li, S Wen, D Wang, X Wang, J Fang, J Cui, B Cheng, Z Wang
J. Exp. Clin. Cancer Res., 2019-07-10;38(1):299.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
T Cell Recruitment to the Intestinal Stem Cell Compartment Drives Immune-Mediated Intestinal Damage after Allogeneic Transplantation
Authors: Ya-Yuan Fu, Anastasiya Egorova, Catherine Sobieski, Jason Kuttiyara, Marco Calafiore, Shuichiro Takashima et al.
Immunity
-
Anti-IL-22 Antibody Attenuates Acute Graft-versus-Host Disease via Increasing Foxp3+ T Cell through Modulation of CD11b+ Cell Function
Authors: J Wu, J Gu, S Zhou, H Lu, Y Lu, L Lu, X Wang
J Immunol Res, 2018-08-07;2018(0):1605341.
Species: Mouse
Sample Types: Whole Cells
Applications: Activation -
CXCL10 increases in human skeletal muscle following damage but is not necessary for muscle regeneration
Authors: MR Deyhle, PS Hafen, J Parmley, CN Preece, M Robison, JR Sorensen, B Jackson, DL Eggett, CR Hancock, RD Hyldahl
Physiol Rep, 2018-04-01;6(8):e13689.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Nitrosative damage during retrovirus infection-induced neuropathic pain
Authors: P Chauhan, WS Sheng, S Hu, S Prasad, JR Lokensgard
J Neuroinflammation, 2018-03-05;15(1):66.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Crucial role of OX40/OX40L signaling in a murine model of asthma
Authors: W Lei, D Zeng, G Liu, Y Zhu, J Wang, H Wu, J Jiang, J Huang
Mol Med Rep, 2018-01-18;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
A Novel C-Terminal Mutation in Gsdma3 (C+/H-) Leads to Alopecia and Corneal Inflammatory Response in Mice
Authors: S Swirski, C Röger, A Pienkowska, C Ihlenburg, G Fischer, O May, M Vorm, M Owczarek-L, J Neidhardt
Invest. Ophthalmol. Vis. Sci., 2018-01-01;59(1):561-571.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Aloperine Protects Mice against DSS-Induced Colitis by PP2A-Mediated PI3K/Akt/mTOR Signaling Suppression
Authors: X Fu, F Sun, F Wang, J Zhang, B Zheng, J Zhong, T Yue, X Zheng, JF Xu, CY Wang
Mediators Inflamm., 2017-09-19;2017(0):5706152.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Novel strategies to mimic transmembrane tumor necrosis factor-dependent activation of tumor necrosis factor receptor 2
Authors: R Fischer, J Marsal, C Guttà, SA Eisler, N Peters, JR Bethea, K Pfizenmaie, RE Kontermann
Sci Rep, 2017-07-26;7(1):6607.
Species: Human
Sample Types: Whole Cells
Applications: Functional Assay -
The excretory-secretory products of Echinococcus granulosus protoscoleces directly regulate the differentiation of B10, B17 and Th17 cells
Authors: W Pan, WT Hao, YJ Shen, XY Li, YJ Wang, FF Sun, JH Yin, J Zhang, RX Tang, JP Cao, KY Zheng
Parasit Vectors, 2017-07-21;10(1):348.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Liver-specific deletion of the Plpp3 gene alters plasma lipid composition and worsens atherosclerosis in apoE−/− mice
Authors: Marco Busnelli, Stefano Manzini, Mika Hilvo, Cinzia Parolini, Giulia S. Ganzetti, Federica Dellera et al.
Scientific Reports
-
Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy
Authors: PG Makker, SS Duffy, JG Lees, CJ Perera, RS Tonkin, O Butovsky, SB Park, D Goldstein, G Moalem-Tay
PLoS ONE, 2017-01-26;12(1):e0170814.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Selective suppression of the ? isoform of p38 MAPK rescues late-stage tau pathology
Alzheimers Res Ther, 2016-12-15;8(1):54.
Species: Mouse
Sample Types: Whole Cells
Applications: ICC -
Temporal Expression of Bim Limits the Development of Agonist-Selected Thymocytes and Skews Their TCR? Repertoire
Authors: David A Hildeman
J. Immunol., 2016-11-16;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Metronomic Doses of Temozolomide Enhance the Efficacy of Carbon Nanotube CpG Immunotherapy in an Invasive Glioma Model.
Authors: Ouyang M, White E, Ren H, Guo Q, Zhang I, Gao H, Yanyan S, Chen X, Weng Y, da Fonseca A, Shah S, Manuel E, Zhang L, Vonderfecht S, Alizadeh D, Berlin J, Badie B
PLoS ONE, 2016-02-01;11(2):e0148139.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Optimizations of siRNA design for the activation of gene transcription by targeting the TATA-box motif.
Authors: Fan, Miaomiao, Zhang, Yijun, Huang, Zhuoqion, Liu, Jun, Guo, Xuemin, Zhang, Hui, Luo, Haihua
PLoS ONE, 2014-09-24;9(9):e108253.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Ninjurin1 deficiency attenuates susceptibility of experimental autoimmune encephalomyelitis in mice.
Authors: Ahn, Bum Ju, Le, Hoang, Shin, Min Wook, Bae, Sung-Jin, Lee, Eun Ji, Wee, Hee-Jun, Cha, Jong-Ho, Lee, Hyo-Jong, Lee, Hye Shin, Kim, Jeong Hu, Kim, Chang-Ye, Seo, Ji Hae, Lo, Eng H, Jeon, Sejin, Lee, Mi-Ni, Oh, Goo Taeg, Yin, Guo Nan, Ryu, Ji-Kan, Suh, Jun-Kyu, Kim, Kyu-Won
J Biol Chem, 2013-12-17;289(6):3328-38.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Regulatory T-cells and cAMP suppress effector T-cells independently of PKA-CREM/ICER: a potential role for Epac.
Authors: Vang, Amanda G, Housley, William, Dong, Hongli, Basole, Chaitali, Ben-Sasson, Shlomo Z, Kream, Barbara, Epstein, Paul M, Clark, Robert B, Brocke, Stefan
Biochem J, 2013-12-15;456(3):463-73.
Species: Mouse
Sample Types: Whole Cells
Applications: Bioassay -
LRIG1 inhibits STAT3-dependent inflammation to maintain corneal homeostasis.
Authors: Nakamura, Takahiro, Hamuro, Junji, Takaishi, Mikiro, Simmons, Szandor, Maruyama, Kazuichi, Zaffalon, Andrea, Bentley, Adam J, Kawasaki, Satoshi, Nagata-Takaoka, Maho, Fullwood, Nigel J, Itami, Satoshi, Sano, Shigetos, Ishii, Masaru, Barrandon, Yann, Kinoshita, Shigeru
J Clin Invest, 2013-12-09;124(1):385-97.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
TGF-beta promotes immune responses in the presence of mesenchymal stem cells.
Authors: Xu C, Yu P, Han X, DU L, Gan J, Wang Y, Shi Y
J Immunol, 2013-11-29;192(1):103-9.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Increased gelatinase B/matrix metalloproteinase 9 (MMP-9) activity in a murine model of acute coxsackievirus B4-induced pancreatitis.
Authors: De Palma AM, Verbeken E, Van Aelst I, Van den Steen PE, Opdenakker G, Neyts J
Virology, 2008-10-16;382(1):20-7.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Identification of a murine CD28 dileucine motif that suppresses single-chain chimeric T-cell receptor expression and function.
Authors: Nguyen P, Moisini I, Geiger TL
Blood, 2003-08-28;102(13):4320-5.
Species: Mouse
Sample Types: Whole Cells
Applications: Functional Assay -
Transcorneal Electrical Stimulation Reduces Neurodegenerative Process in a Mouse Model of Glaucoma.
Authors: Jassim, A H, Cavanaugh, M Et al.
Ann Biomed Eng
-
Discovery and characterization of a monoclonal antibody targeting a conformational epitope of IL-6/IL-6R alpha to inhibit IL-6/ IL-6R alpha /gp130 hexameric signaling complex formation
Authors: Chun-Chi Chou, Kuo-Tai Hua, Min-Wei Chen, Chin-Jui Wu, Chun-Hua Hsu, Jann-Tay Wang et al.
mAbs
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Mouse CD3 Antibody
Average Rating: 5 (Based on 5 Reviews)
Have you used Mouse CD3 Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Reason for Rating: The antibody CD3 works excellently. It labels leukocytes trafficked to inflamed tissue areas in mouse spinal cord in the EAE model of Multiple Sclerosis Sample Tested: Spinal cord tissue Species: Mouse
Green fluorescent is CD3 in mice tumors.
Permeabilisation - 30mins at RT
Block -1% BSA - 30mins at RT
Primary Ab - diluted in block - 1 in 25 - for O/N at 4'C
Secondary ab - diluted in block - 2hrs at RT