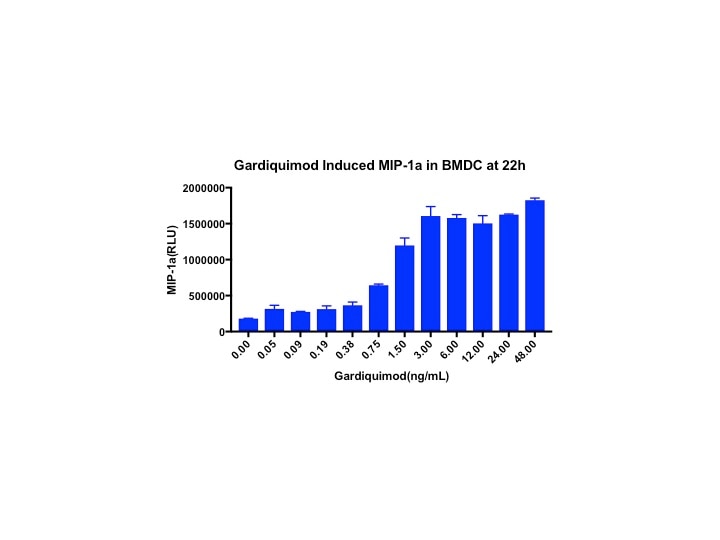
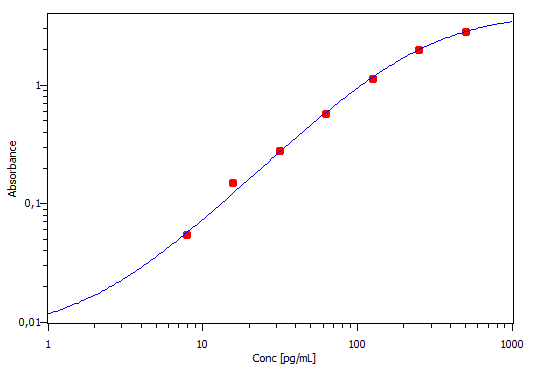
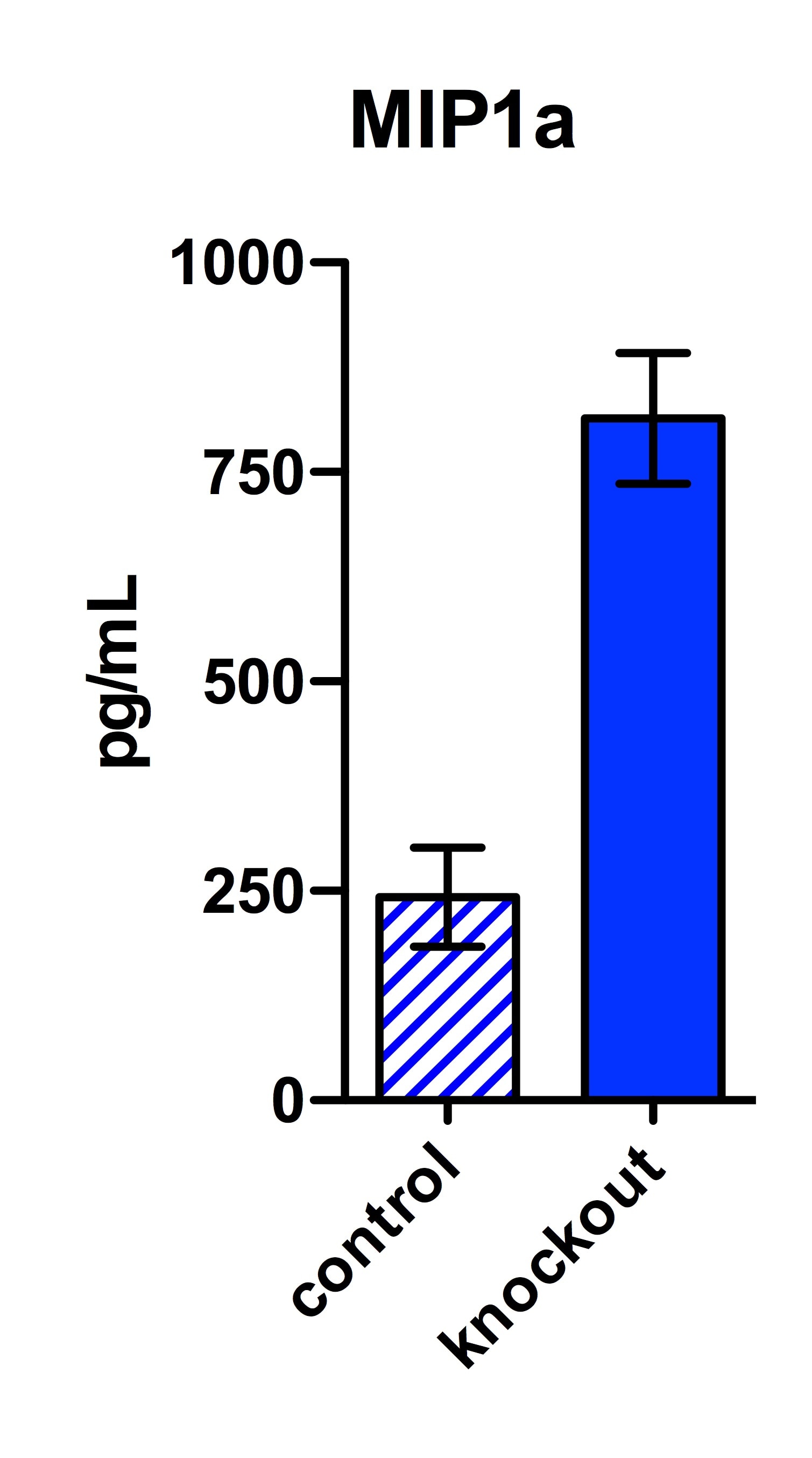
Mouse CCL3/MIP-1 alpha DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse CCL3/MIP-1alpha. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: CCL3/MIP-1 alpha
MIP-1 alpha (macrophage inflammatory protein 1 alpha) is a member of the CC or beta chemokine subfamily that was originally purified from the conditioned media of an LPS-stimulated murine macrophage cell line. MIP-1 alpha acts as a chemoattractant to a variety of cells including monocytes, T cells, B cells and eosinophils.
The two human MIP-1 alpha genes arise by duplication/mutation. They code for MIP-1 alpha isoforms CCL3/LD78a and CCL3L1/LD78b, which share 94% amino acid sequence homology. Whereas the human CCL3/LD78a is a single-copy gene, the human CCL3L1/LD78b gene copy number varies within the population. Human CCL3L1/LD78b binds and signals through chemokine receptors CCR1, CCR5. When compared to CCL3/LD78a, CCL3L1/LD78b has higher binding affinity to CCR5, which also functions as a coreceptor for HIV-1 entry. The copy number of CCL3L1 is one of several genetic determinants of HIV-1 susceptibility.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse CCL3/MIP-1 alpha DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
52
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Anti-Neuroinflammatory Effects of Ginkgo biloba Extract EGb 761 in LPS-Activated BV2 Microglial Cells
Authors: Sun, L;Apweiler, M;Tirkey, A;Klett, D;Normann, C;Dietz, GPH;Lehner, MD;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Anti-Neuroinflammatory Effects of a Macrocyclic Peptide-Peptoid Hybrid in Lipopolysaccharide-Stimulated BV2 Microglial Cells
Authors: Sun, L;Wilke Saliba, S;Apweiler, M;Akmermer, K;Herlan, C;Grathwol, C;de Oliveira, ACP;Normann, C;Jung, N;Bräse, S;Fiebich, BL;
International journal of molecular sciences
Species: Mouse
Sample Types: Cell Culture Supernates
-
Highly secreted tryptophanyl tRNA synthetase 1 as a potential theranostic target for hypercytokinemic severe sepsis
Authors: Kim, YT;Huh, JW;Choi, YH;Yoon, HK;Nguyen, TT;Chun, E;Jeong, G;Park, S;Ahn, S;Lee, WK;Noh, YW;Lee, KS;Ahn, HS;Lee, C;Lee, SM;Kim, KS;Suh, GJ;Jeon, K;Kim, S;Jin, M;
EMBO molecular medicine
Species: Mouse
Sample Types: Plasma, Peritoneal Lavage Fluid
-
Coenzyme Q10 exhibits anti-inflammatory and immune-modulatory thereby decelerating the occurrence of experimental cerebral malaria
Authors: Nyariki, JN;Kimani, NM;Kibet, PS;Kinuthia, GK;Isaac, AO;
Molecular and biochemical parasitology
Species: Mouse
Sample Types: Tissue Homogenates
-
The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion
Authors: N Zeren, Z Afzal, S Morgan, G Marshall, M Uppiliappa, J Merritt, SJ Coniglio
International Journal of Molecular Sciences, 2023-03-07;24(6):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Tryptophan-dependent and -independent secretions of tryptophanyl- tRNA synthetase mediate innate inflammatory responses
Authors: TTT Nguyen, YH Choi, WK Lee, Y Ji, E Chun, YH Kim, JE Lee, HS Jung, JH Suh, S Kim, M Jin
Cell Reports, 2022-12-30;42(1):111905.
Species: Mouse
Sample Types: Lavage Fluid
-
AhR Mediated Activation of Pro-Inflammatory Response of RAW 264.7 Cells Modulate the Epithelial-Mesenchymal Transition
Authors: P Selvam, CM Cheng, HU Dahms, VK Ponnusamy, YY Sun
Toxics, 2022-10-27;10(11):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
cis interaction of CD153 with TCR/CD3 is crucial for the pathogenic activation of senescence-associated T�cells
Authors: Y Fukushima, K Sakamoto, M Matsuda, Y Yoshikai, H Yagita, D Kitamura, M Chihara, N Minato, M Hattori
Cell Reports, 2022-09-20;40(12):111373.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Phosphatidylinositol 3-Kinase (PI3K) Orchestrates Aspergillus fumigatus-Induced Eosinophil Activation Independently of Canonical Toll-Like Receptor (TLR)/C-Type-Lectin Receptor (CLR) Signaling
Authors: A Dietschman, S Schruefer, S Westermann, F Henkel, K Castiglion, R Willebrand, J Adam, J Ruland, R Lang, DC Sheppard, J Esser-von-, D Radtke, S Krappmann, D Voehringer
MBio, 2022-06-13;0(0):e0123922.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Kupffer Cells and Blood Monocytes Orchestrate the Clearance of Iron-Carbohydrate Nanoparticles from Serum
Authors: T Arsiwala, AS Vogt, AE Barton, V Manolova, F Funk, B Flühmann, MF Bachmann
International Journal of Molecular Sciences, 2022-02-28;23(5):.
Species: Mouse
Sample Types: Tissue Lysates
-
Signaling through Syk or CARD9 Mediates Species-Specific Anti-Candida Protection in Bone Marrow Chimeric Mice
Authors: E Zajta, K Csonka, A Tóth, L Tiszlavicz, T Németh, A Orosz, Á Novák, M Csikós, C Vágvölgyi, A Mócsai, A Gácser
MBio, 2021-08-31;12(4):e0160821.
Species: Mouse, Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Resveratrol-Loaded Lipid-Core Nanocapsules Modulate Acute Lung Inflammation and Oxidative Imbalance Induced by LPS in Mice
Authors: MTP de Oliveir, DS Coutinho, SS Guterres, AR Pohlmann, PMRE Silva, MA Martins, A Bernardi
Pharmaceutics, 2021-05-10;13(5):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oligodeoxynucleotides containing unmethylated cytosine-guanine motifs are effective immunostimulants against pneumococcal meningitis in the immunocompetent and neutropenic host
Authors: S Ribes, L Zacke, S Nessler, N Saiepour, E Avendaño-G, M Ballüer, UK Hanisch, R Nau
Journal of Neuroinflammation, 2021-02-02;18(1):39.
Species: Mouse
Sample Types: Tissue Homogenates
-
NR4A nuclear receptors restrain B cell responses to antigen when second signals are absent or limiting
Authors: C Tan, R Hiwa, JL Mueller, V Vykunta, K Hibiya, M Noviski, J Huizar, JF Brooks, J Garcia, C Heyn, Z Li, A Marson, J Zikherman
Nat. Immunol., 2020-08-31;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interleukin-1 Receptor-Induced Nitric Oxide Production in the Pancreas Controls Hyperglycemia Caused by Scorpion Envenomation
Authors: MB Reis, J Elias-Oliv, MR Pastore, SG Ramos, LG Gardinassi, LH Faccioli
Toxins (Basel), 2020-03-05;12(3):.
Species: Mouse
Sample Types: Serum
-
Pre-treatment with the viral Toll-like receptor 3 agonist poly(I:C) modulates innate immunity and protects neutropenic mice infected intracerebrally with Escherichia coli
Authors: S Ribes, C Arcilla, M Ott, S Schütze, UK Hanisch, S Nessler, R Nau
J Neuroinflammation, 2020-01-17;17(1):24.
Species: Mouse
Sample Types: Tissue Hom
-
Pseudomonas aeruginosa Lipoxygenase LoxA Contributes to Lung Infection by Altering the Host Immune Lipid Signaling
Authors: E Morello, T Pérez-Bere, C Boisseau, T Baranek, A Guillon, D Bréa, P Lanotte, X Carpena, N Pietrancos, V Hervé, R Ramphal, N Cenac, M Si-Tahar
Front Microbiol, 2019-08-14;10(0):1826.
Species: Mouse
Sample Types: BALF
-
In-depth characterization of congenital Zika syndrome in immunocompetent mice: Antibody-dependent enhancement and an antiviral peptide therapy
Authors: VN Camargos, G Foureaux, DC Medeiros, VT da Silveir, CM Queiroz-Ju, ALB Matosinhos, AFA Figueiredo, CDF Sousa, TP Moreira, VF Queiroz, ACF Dias, KTO Santana, I Passos, ALCV Real, LC Silva, FAG Mourão, NT Wnuk, MAP Oliveira, S Macari, T Silva, GP Garlet, JA Jackman, FM Soriani, MFD Moraes, EMAM Mendes, FM Ribeiro, GMJ Costa, AL Teixeira, NJ Cho, ACP Oliveira, MM Teixeira, VV Costa, DG Souza
EBioMedicine, 2019-05-23;0(0):.
Species: Mouse
Sample Types: Plasma
-
New role of P2X7 receptor in an Alzheimer's disease mouse model
Authors: E Martin, M Amar, C Dalle, I Youssef, C Boucher, C Le Duigou, M Brückner, A Prigent, V Sazdovitch, A Halle, JM Kanellopou, B Fontaine, B Delatour, C Delarasse
Mol. Psychiatry, 2018-06-22;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Murine neutrophils treated with alphaB-crystallin reduce IL-12p40 production by dendritic cells
Authors: TM Finlay, AL Palmer, SS Ousman
Immunology, 2018-04-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Surface layer proteins from virulent Clostridium difficile ribotypes exhibit signatures of positive selection with consequences for innate immune response
Authors: M Lynch, TA Walsh, I Marszalows, AE Webb, M MacAogain, TR Rogers, H Windle, D Kelleher, MJ O'Connell, CE Loscher
BMC Evol. Biol, 2017-03-23;17(1):90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The Carbohydrate Lectin Receptor, Dectin-1, Mediates Immune Response to Exserohilum rostratum
Infect. Immun, 2017-02-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory FADDosome Complex upon TRAIL Stimulation
Authors: CM Henry, SJ Martin
Mol. Cell, 2017-02-16;65(4):715-729.e5.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Deletion of the Chemokine Binding Protein Gene from the Parapoxvirus Orf Virus Reduces Virulence and Pathogenesis in Sheep
Authors: SB Fleming, C McCaughan, Z Lateef, A Dunn, LM Wise, NC Real, AA Mercer
Front Microbiol, 2017-01-24;8(0):46.
Species: Mouse
Sample Types: Protein
-
C1q Modulates the Response to TLR7 Stimulation by Pristane-Primed Macrophages: Implications for Pristane-Induced Lupus.
Authors: Carlucci F, Ishaque A, Ling G, Szajna M, Sandison A, Donatien P, Cook H, Botto M
J Immunol, 2016-01-15;196(4):1488-94.
Species: Mouse
Sample Types: Serum
-
The transcription factor ATF7 mediates lipopolysaccharide-induced epigenetic changes in macrophages involved in innate immunological memory.
Authors: Yoshida K, Maekawa T, Zhu Y, Renard-Guillet C, Chatton B, Inoue K, Uchiyama T, Ishibashi K, Yamada T, Ohno N, Shirahige K, Okada-Hatakeyama M, Ishii S
Nat Immunol, 2015-08-31;16(10):1034-43.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Ethanolic Echinacea purpurea Extracts Contain a Mixture of Cytokine-Suppressive and Cytokine-Inducing Compounds, Including Some That Originate from Endophytic Bacteria.
Authors: Todd D, Gulledge T, Britton E, Oberhofer M, Leyte-Lugo M, Moody A, Shymanovich T, Grubbs L, Juzumaite M, Graf T, Oberlies N, Faeth S, Laster S, Cech N
PLoS ONE, 2015-05-01;10(5):e0124276.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A critical role for the TLR signaling adapter Mal in alveolar macrophage-mediated protection against Bordetella pertussis.
Authors: Bernard N, Finlay C, Tannahill G, Cassidy J, O'Neill L, Mills K
Mucosal Immunol, 2014-12-17;8(5):982-92.
Species: Mouse
Sample Types: Tissue Homogenates
-
Murine lung injury caused by Leptospira interrogans glycolipoprotein, a specific Na/K-ATPase inhibitor.
Authors: Goncalves-de-Albuquerque C, Burth P, Silva A, de Moraes I, Oliveira F, Santelli R, Freire A, de Lima G, da Silva E, da Silva C, Morandi V, Bozza P, Younes-Ibrahim M, de Castro Faria Neto H, de Castro Faria M
Respir Res, 2014-08-14;15(0):93.
Species: Mouse
Sample Types: BALF
-
Intraperitoneal prophylaxis with CpG oligodeoxynucleotides protects neutropenic mice against intracerebral Escherichia coli K1 infection.
Authors: Ribes S, Meister T, Ott M, Redlich S, Janova H, Hanisch U, Nessler S, Nau R
J Neuroinflammation, 2014-01-23;11(0):14.
Species: Mouse
Sample Types: Tissue Homogenates
-
Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion.
Authors: Menezes-Garcia Z, Oliveira M, Lima R, Soriani F, Cisalpino D, Botion L, Teixeira M, Souza D, Ferreira A
Obesity (Silver Spring), 2013-12-14;22(3):663-72.
Species: Mouse
Sample Types: Tissue Homogenates
-
UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d.
Authors: Ryser S, Schuppli M, Gauthier B, Hernandez D, Roye O, Hohl D, German B, Holzwarth J, Moodycliffe A
J Invest Dermatol, 2013-07-18;134(1):192-202.
Species: Mouse
Sample Types: Tissue Homogenates
-
A novel murine model of rhinoscleroma identifies Mikulicz cells, the disease signature, as IL-10 dependent derivatives of inflammatory monocytes.
Authors: Fevre C, Almeida A, Taront S, Pedron T, Huerre M, Prevost M, Kieusseian A, Cumano A, Brisse S, Sansonetti P, Tournebize R
EMBO Mol Med, 2013-04-01;5(4):516-30.
Species: Mouse
Sample Types: Tissue Homogenates
-
Syntaxin binding protein 1 is not required for allergic inflammation via IgE-mediated mast cell activation.
Authors: Wu Z, MacNeil A, Berman J, Lin T
PLoS ONE, 2013-03-06;8(3):e58560.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection.
Authors: Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C
Infect. Immun., 2011-10-24;80(1):100-9.
Species: Mouse
Sample Types: Serum
-
Mucosal delivery of human papillomavirus pseudovirus-encapsidated plasmids improves the potency of DNA vaccination.
Authors: Graham BS, Kines RC, Corbett KS, Nicewonger J, Roberts JN, Schiller JT, Buck CB
Mucosal Immunol, 2010-06-16;3(5):475-86.
Species: Mouse
Sample Types: BALF
-
Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid.
Authors: van Neerven S, Regen T, Wolf D, Nemes A, Johann S, Beyer C, Hanisch UK, Mey J
J. Neurochem., 2010-06-16;114(5):1511-26.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chorionic gonadotropin alleviates thioglycollate-induced peritonitis by affecting macrophage function.
Authors: Wan H, Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Van Rooijen N, Khan NA, Kiekens RC, Benner R, Versnel MA
J. Leukoc. Biol., 2009-05-04;86(2):361-70.
Species: Mouse
Sample Types: Peritoneal Lavage Fluid
-
CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process.
Authors: Wu Y, Li YY, Matsushima K, Baba T, Mukaida N
J. Immunol., 2008-11-01;181(9):6384-93.
Species: Mouse
Sample Types: Tissue Homogenates
-
gammadeltaT cell-mediated regulation of chemokine producing macrophages during Listeria monocytogenes infection-induced inflammation.
Authors: Tramonti D, Rhodes K, Martin N, Dalton JE, Andrew E, Carding SR
J. Pathol., 2008-10-01;216(2):262-70.
Species: Mouse
Sample Types: Cell Culture Supernates
-
VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways.
Authors: Tiwari N, Wang CC, Brochetta C, Ke G, Vita F, Qi Z, Rivera J, Soranzo MR, Zabucchi G, Hong W, Blank U
Blood, 2008-01-18;111(7):3665-74.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection.
Authors: Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC
J. Immunol., 2008-01-15;180(2):1080-7.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes.
Authors: Arias MA, Jaramillo G, Lopez YP, Mejia N, Mejia C, Pantoja AE, Shattock RJ, Garcia LF, Griffin GE
J. Immunol., 2007-12-15;179(12):8381-91.
Species: Human
Sample Types: Cell Culture Supernates
-
CD8+ T Cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice.
Authors: Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD
J. Immunol., 2007-06-15;178(12):8090-6.
Species: Mouse
Sample Types: BALF
-
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity.
Authors: Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ
Immunity, 2007-04-12;26(4):445-59.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis.
Authors: Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR
Eur. J. Immunol., 2006-07-01;36(7):1729-38.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Effects of dexamethazone on LPS-induced activationand migration of mouse dendritic cells revealed by a genome-wide transcriptional analysis.
Authors: Vizzardelli C, Pavelka N, Luchini A, Zanoni I, Bendickson L, Pelizzola M, Beretta O, Foti M, Granucci F, Nilsen-Hamilton M, Ricciardi-Castagnoli P
Eur. J. Immunol., 2006-06-01;36(6):1504-15.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Mucosal administration of CpG oligodeoxynucleotide elicits strong CC and CXC chemokine responses in the vagina and serves as a potent Th1-tilting adjuvant for recombinant gD2 protein vaccination against genital herpes.
Authors: Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM
J. Virol., 2006-06-01;80(11):5283-91.
Species: Mouse
Sample Types: Tissue Homogenates
-
Differential involvement of TLR2 and TLR4 in host survival during pulmonary infection with Chlamydia pneumoniae.
Authors: Rodriguez N, Wantia N, Fend F, Durr S, Wagner H, Miethke T
Eur. J. Immunol., 2006-05-01;36(5):1145-55.
Species: Mouse
Sample Types: Tissue Homogenates
-
Immunosuppressive effects of CCL17 on pulmonary antifungal responses during pulmonary invasive aspergillosis.
Authors: Carpenter KJ, Hogaboam CM
Infect. Immun., 2005-11-01;73(11):7198-207.
Species: Mouse
Sample Types: Tissue Homogenates
-
MIP-1 gamma promotes receptor-activator-of-NF-kappa-B-ligand-induced osteoclast formation and survival.
Authors: Okamatsu Y, Battaglino R, Spate U, Stashenko P
J. Immunol., 2004-08-01;173(3):2084-90.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Specific engagement of TLR4 or TLR3 does not lead to IFN-beta-mediated innate signal amplification and STAT1 phosphorylation in resident murine alveolar macrophages.
Authors: Punturieri A, Alviani RS, Polak T, Copper P, Sonstein J, Curtis JL
J. Immunol., 2004-07-15;173(2):1033-42.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse CCL3/MIP-1 alpha DuoSet ELISA
Average Rating: 5 (Based on 5 Reviews)
Have you used Mouse CCL3/MIP-1 alpha DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
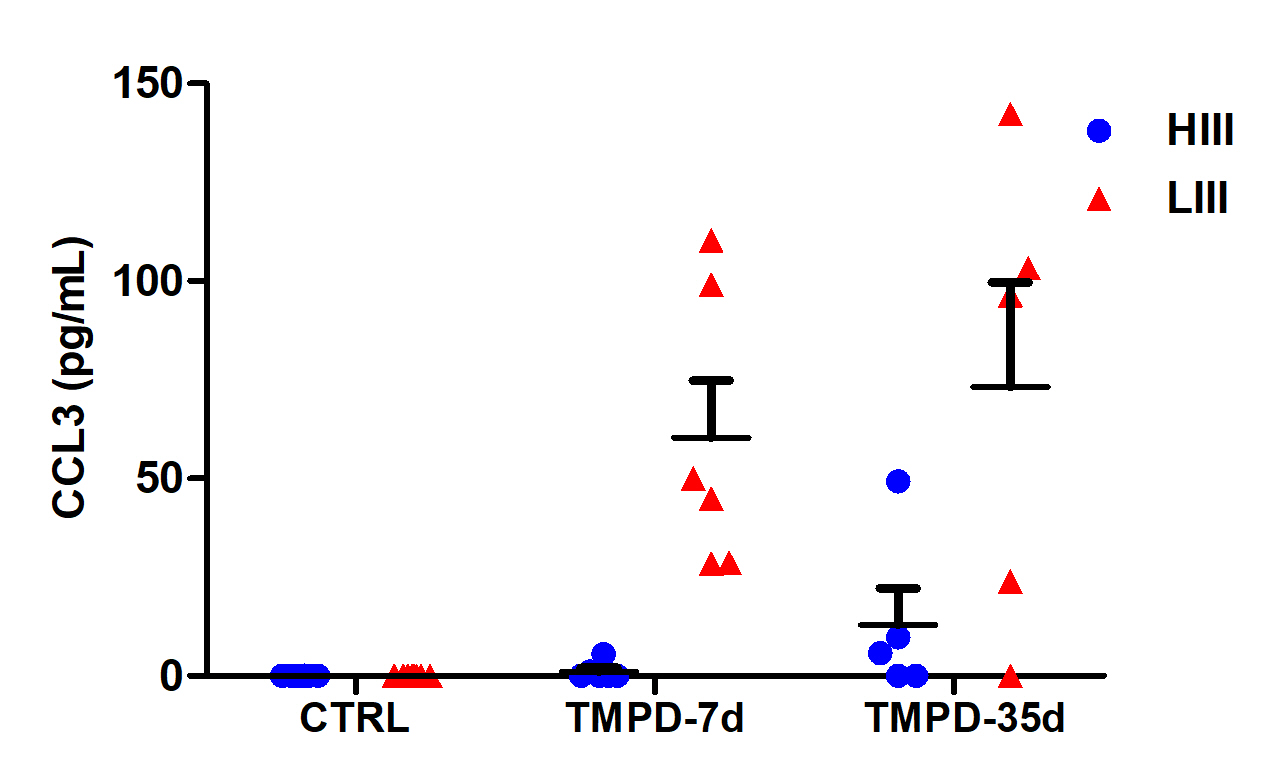
Mice (HIII and LIII strains) injected i.p. with 2,4,6,10 tetramethylpentadecane (pristane).