Human VE-Cadherin Antibody Summary
Asp48-Gln593
Accession # P33151
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human VE‑Cadherin by Western Blot. Western blot shows lysate of HUVEC human umbilical vein endothelial cells. PVDF membrane was probed with 0.25 µg/mL of Goat Anti-Human VE-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF938) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for VE-Cadherin at approximately 125 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
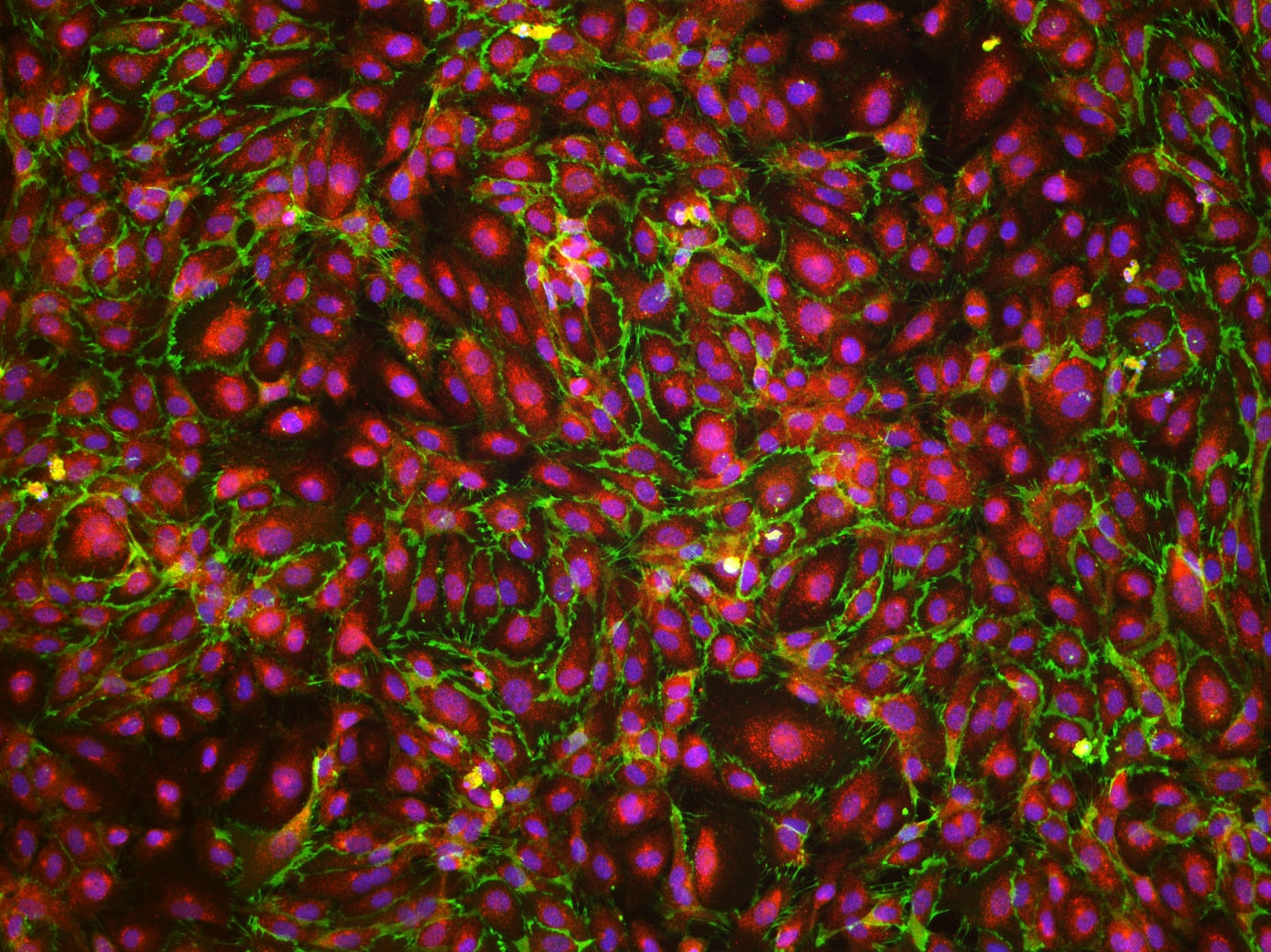
VE‑Cadherin in HUVEC Cells. VE-Cadherin was detected in immersion fixed HUVEC human umbilical vein endothelial cells using Goat Anti-Human VE-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF938) at 10 µg/mL for 3 hours at room temperature. Cells were stained using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). Specific staining was localized to the plasma membrane. View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
Detection of Human VE‑Cadherin by Simple WesternTM. Simple Western lane view shows lysates of HUVEC human umbilical vein endothelial cells, loaded at 0.2 mg/mL. A specific band was detected for VE‑Cadherin at approximately 162 kDa (as indicated) using 12.5 µg/mL of Goat Anti-Human VE‑Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF938). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: VE-Cadherin
Vascular endothelial (VE) - cadherin (VE-CAD), also called 7B4 and cadherin-5, is a member of the cadherin family of cell adhesion molecules. Cadherins are
calcium‑dependent transmembrane proteins which bind to one another in a homophilic manner. On their cytoplasmic side, they associate with the three catenins, alpha, beta, and gamma (plakoglobin). This association links the cadherin protein to the cytoskeleton. Without association with the catenins, the cadherins are non-adhesive. Cadherins play a role in development, specifically in tissue formation. They may also help to maintain tissue architecture in the adult. VE-Cadherin has been shown to play important roles in vasculogenesis and angiogenesis. VE-cadherin is a classical cadherin molecule. Classical cadherins consist of a large extracellular domain which contains DXD and DXNDN repeats responsible for mediating calcium‑dependent adhesion, a single-pass transmembrane domain, and a short carboxy-terminal cytoplasmic domain responsible for interacting with the catenins. Human VE-cadherin is a 784 amino acid (aa) residue protein with a 25 aa signal sequence and a 759 aa propeptide. The mature protein begins at amino acid 48 and has a 546 aa extracellular domain, a 27 aa transmembrane domain, and a 164 aa cytoplasmic domain. The human and mouse mature VE-CAD proteins share approximately 74% homology.
- Shimoyama, Y. et al. (1989) J. Cell Biol. 109:1787.
- Bussemakers, M.J.G. et al. (1993) Mol. Biol. Reports 17:123.
- Overduin, M. et al. (1995) Science 267:386.
- Takeichi, M. (1991) Science 251:1451.
- Nose, A. et al. (1987) EMBO J. 6:3655.
- Carmeliet, P. et al. (1999) Cell 98:147.
- Gory-Faure, S. et al. (1999) Development 126:2093.
Product Datasheets
Citations for Human VE-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
63
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Engineering a 3D Vascularized Adipose Tissue Construct Using a Decellularized Lung Matrix
Authors: Megan K. DeBari, Wai Hoe Ng, Mallory D. Griffin, Lauren E. Kokai, Kacey G. Marra, J. Peter Rubin et al.
Biomimetics (Basel)
-
PAR ‐3 controls endothelial planar polarity and vascular inflammation under laminar flow
Authors: Takao Hikita, Fatemeh Mirzapourshafiyi, Pedro Barbacena, Meghan Riddell, Ayesha Pasha, Mengnan Li et al.
EMBO reports
-
von Willebrand Factor Is Produced Exclusively by Endothelium, Not Neointima, in Occlusive Vascular Lesions in Both Pulmonary Hypertension and Atherosclerosis
Authors: Lea C. Steffes, Paul Cheng, Thomas Quertermous, Maya E. Kumar
Circulation
-
Connexin 37 sequestering of activated-ERK in the cytoplasm promotes p27-mediated endothelial cell cycle arrest
Authors: Acharya BR, Fang JS, Jeffery ED et al.
Life science alliance
-
A small molecule-based strategy for endothelial differentiation and three-dimensional morphogenesis from human embryonic stem cells
Authors: Yijie Geng, Bradley Feng
Heliyon
-
Unlocking the Role of Endothelial MPL Receptor and JAK2V617F Mutation: Insights into Cardiovascular Dysfunction in MPNs and CHIP
Authors: Zhang, H;Kafeiti, N;Lee, S;Masarik, K;Zheng, H;Zhan, H;
bioRxiv : the preprint server for biology
-
The pro-inflammatory response to influenza A virus infection is fueled by endothelial cells
Authors: Lisa Bauer, Laurine C Rijsbergen, Lonneke Leijten, Feline FW Benavides, Danny Noack, Mart M Lamers et al.
Life Science Alliance
-
Id genes are essential for early heart formation
Authors: Thomas J. Cunningham, Michael S. Yu, Wesley L. McKeithan, Sean Spiering, Florent Carrette, Chun-Teng Huang et al.
Genes & Development
-
Efficient Gene Editing at Major CFTR Mutation Loci
Authors: Jinxue Ruan, Hiroyuki Hirai, Dongshan Yang, Linyuan Ma, Xia Hou, Hong Jiang et al.
Molecular Therapy - Nucleic Acids
-
Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids
Authors: Nikoli? MZ, Caritg O, Jeng Q et al.
Elife
-
Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration
Authors: Joana R Carvalho, Isabela C Fortunato, Catarina G Fonseca, Anna Pezzarossa, Pedro Barbacena, Maria A Dominguez-Cejudo et al.
eLife
-
Neuropilin-1 interacts with VE-cadherin and TGFBR2 to stabilize adherens junctions and prevent activation of endothelium under flow
Authors: Emy Bosseboeuf, Anissa Chikh, Ahmed Bey Chaker, Tom P. Mitchell, Dhilakshani Vignaraja, Ridhi Rajendrakumar et al.
Science Signaling
-
Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels
Authors: Matthew R. Zanotelli, Hamisha Ardalani, Jue Zhang, Zhonggang Hou, Eric H. Nguyen, Scott Swanson et al.
Acta Biomaterialia
-
Specification of fetal liver endothelial progenitors to functional zonated adult sinusoids requires c-Maf induction
Authors: Jesus Maria Gómez-Salinero, Franco Izzo, Yang Lin, Sean Houghton, Tomer Itkin, Fuqiang Geng et al.
Cell Stem Cell
-
Endothelial Cell Mediated Promotion of Ciliated Cell Differentiation of Human Airway Basal Cells via Insulin and Insulin-Like Growth Factor 1 Receptor Mediated Signaling.
Authors: Gomi K, Tang Y, Arbelaez V et al.
Stem Cell Rev.
-
Glycolysis is integral to histamine‐induced endothelial hyperpermeability
Authors: Athanasios Ziogas, Md Sanaullah Sajib, Jong‐Hyung Lim, Tiago C. Alves, Anupam Das, Anke Witt et al.
The FASEB Journal
-
Multi-targeted action of rooibos may protect against ischaemic stroke-induced neurological deficit and endothelial dysfunction
Authors: Pretorius, L;Ross, KS;Smith, C;
Journal of ethnopharmacology
Species: Zebrafish
Sample Types: Whole Tissue
Applications: Immunohistochemistry -
c-Src-induced vascular malformations require localised matrix degradation at focal adhesions
Authors: Essebier, P;Keyser, M;Yordanov, T;Hill, B;Yu, A;Noordstra, I;Yap, AS;Stehbens, SJ;Lagendijk, AK;Schimmel, L;Gordon, EJ;
Journal of cell science
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
A novel efficient strategy to generate liver sinusoidal endothelial cells from human pluripotent stem cells
Authors: Tian, SP;Ge, JY;Song, YM;Yu, XQ;Chen, WH;Chen, YY;Ye, D;Zheng, YW;
Scientific reports
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
ZO-1 interacts with YB-1 in endothelial cells to regulate stress granule formation during angiogenesis
Authors: El Bakkouri, Y;Chidiac, R;Delisle, C;Corriveau, J;Cagnone, G;Gaonac'h-Lovejoy, V;Chin, A;Lécuyer, É;Angers, S;Joyal, JS;Topisirovic, I;Hulea, L;Dubrac, A;Gratton, JP;
Nature communications
Species: Bovine
Sample Types: cell line
Applications: IF/ICC, Westen Blot -
Regulation of angiogenesis by endocytic trafficking mediated by cytoplasmic dynein 1 light intermediate chain 1
Authors: Johnson, D;Colijn, S;Richee, J;Yano, J;Burns, M;Davis, AE;Pham, VN;Saric, A;Jain, A;Yin, Y;Castranova, D;Melani, M;Fujita, M;Grainger, S;Bonifacino, JS;Weinstein, BM;Stratman, AN;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
CXCR3-CXCL11 signaling restricts angiogenesis and promotes pericyte recruitment
Authors: Goeckel, ME;Lee, J;Levitas, A;Colijn, S;Mun, G;Burton, Z;Chintalapati, B;Yin, Y;Abello, J;Stratman, A;
bioRxiv : the preprint server for biology
Species: Fish - Danio rerio (Zebrafish)
Sample Types: Whole Cells
Applications: Immunocytochemistry/ Immunofluorescence -
Engineered 3D Immuno-Glial-Neurovascular Human Brain Model
Authors: Stanton, AE;Bubnys, A;Agbas, E;James, B;Park, DS;Jiang, A;Pinals, RL;Truong, N;Loon, A;Staab, C;Liu, L;Cerit, O;Wen, HL;Kellis, M;Blanchard, JW;Langer, RS;Tsai, LH;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
SARS-CoV-2 infection of human pluripotent stem cell-derived vascular cells reveals smooth muscle cells as key mediators of vascular pathology during infection
Authors: Richards, A;Khalil, A;Friesen, M;Whitfield, TW;Lungjangwa, T;Gehrke, L;Mooney, D;Jaenisch, R;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Argininosuccinate lyase deficiency causes blood-brain barrier disruption via nitric oxide-mediated dysregulation of claudin expression
Authors: Kho, J;Polak, U;Jiang, MM;Odom, JD;Hunter, JV;Ali, SM;Burrage, LC;Nagamani, SC;Pautler, RG;Thompson, HP;Urayama, A;Jin, Z;Lee, B;
JCI insight
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Polarized Mechanosensitive Signaling Domains Protect Arterial Endothelial Cells Against Inflammation
Authors: Hong, SG;Ashby, JW;Kennelly, JP;Wu, M;Chattopadhyay, E;Foreman, R;Tontonoz, P;Turowski, P;Gallagher-Jones, M;Mack, JJ;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry -
Co-culture models of endothelial cells, macrophages, and vascular smooth muscle cells for the study of the natural history of atherosclerosis
Authors: M Liu, S Samant, CH Vasa, RM Pedrigi, UM Oguz, S Ryu, T Wei, DR Anderson, DK Agrawal, YS Chatzizisi
PLoS ONE, 2023-01-20;18(1):e0280385.
Species: Human
Sample Types: Whole, Whole Cells
Applications: ICC -
NOTCH3 drives meningioma tumorigenesis and resistance to radiotherapy
Authors: Choudhury, A;Cady, M;Lucas, C;Najem, H;Phillips, J;Palikuqi, B;Zakimi, N;Joseph, T;Birrueta, J;Chen, W;Bush, N;Hervey-Jumper, S;Klein, O;Toedebusch, C;Horbinski, C;Magill, S;Bhaduri, A;Perry, A;Dickinson, P;Heimberger, A;Ashworth, A;Crouch, E;Raleigh, D;
bioRxiv
Species: Human
Sample Types: Whole Tissue
Applications: RNAScope -
Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin
Authors: H Kim, K Leng, J Park, AG Sorets, S Kim, A Shostak, RJ Embalabala, K Mlouk, KA Katdare, IVL Rose, SM Sturgeon, EH Neal, Y Ao, S Wang, MV Sofroniew, JM Brunger, DG McMahon, MS Schrag, M Kampmann, ES Lippmann
Nature Communications, 2022-11-02;13(1):6581.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Nascent polypeptide-Associated Complex and Signal Recognition Particle have cardiac-specific roles in heart development and remodeling
Authors: AM Schroeder, T Nielsen, M Lynott, G Vogler, AR Colas, R Bodmer
PloS Genetics, 2022-10-14;18(10):e1010448.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Murine endothelial serine palmitoyltransferase 1 (SPTLC1) is required for vascular development and systemic sphingolipid homeostasis
Authors: Kuo A, Checa A, Niaudet C et al.
eLife
-
Peristaltic pumps adapted for laminar flow experiments enhance in vitro modeling of vascular cell behavior
Authors: J Abello, S Raghavan, YY Yien, AN Stratman
The Journal of Biological Chemistry, 2022-08-19;0(0):102404.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
LDLR expression in the cochlea suggests a role in endolymph homeostasis and cochlear amplification
Authors: A Saume, M Thiry, J Defourny
Hearing research, 2021-07-15;409(0):108311.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Mechanisms of angiogenic incompetence in Hutchinson-Gilford progeria via downregulation of endothelial NOS
Authors: YG Gete, LW Koblan, X Mao, M Trappio, B Mahadik, JP Fisher, DR Liu, K Cao
Aging Cell, 2021-06-04;0(0):e13388.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: IHC, Western Blot -
Genetic disruption of the Blood Brain Barrier leads to protective barrier formation at the Glia Limitans
Authors: P Mora, PL Hollier, S Guimbal, A Abelanet, A Diop, L Cornuault, T Couffinhal, S Horng, AP Gadeau, MA Renault, C Chapouly
PLoS Biol, 2020-11-30;18(11):e3000946.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Effect of Enzymatically Extracted Fucoidans on Angiogenesis and Osteogenesis in Primary Cell Culture Systems Mimicking Bone Tissue Environment
Authors: J Ohmes, Y Xiao, F Wang, MD Mikkelsen, TT Nguyen, H Schmidt, A Seekamp, AS Meyer, S Fuchs
Mar Drugs, 2020-09-21;18(9):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes
Authors: JW Blanchard, M Bula, J Davila-Vel, LA Akay, L Zhu, A Frank, MB Victor, JM Bonner, H Mathys, YT Lin, T Ko, DA Bennett, HP Cam, M Kellis, LH Tsai
Nat. Med., 2020-06-08;26(6):952-963.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Identification of a combination of transcription factors that synergistically increases endothelial cell barrier resistance
Authors: F Roudnicky, BK Kim, Y Lan, R Schmucki, V Küppers, K Christense, M Graf, C Patsch, M Burcin, CA Meyer, PD Westenskow, CA Cowan
Sci Rep, 2020-03-03;10(1):3886.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
aPKC controls endothelial growth by modulating c-Myc via FoxO1 DNA-binding ability
Authors: M Riddell, A Nakayama, T Hikita, F Mirzapours, T Kawamura, A Pasha, M Li, M Masuzawa, M Looso, T Steinbache, K Ebnet, M Potente, T Hirose, S Ohno, I Fleming, S Gattenlöhn, PP Aung, T Phung, O Yamasaki, T Yanagi, H Umemura, M Nakayama
Nat Commun, 2018-12-17;9(1):5357.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation
Authors: K Nonomura, V Lukacs, DT Sweet, LM Goddard, A Kanie, T Whitwam, SS Ranade, T Fujimori, ML Kahn, A Patapoutia
Proc. Natl. Acad. Sci. U.S.A., 2018-11-27;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-Fr -
Vascular CXCR4 expression promotes vessel sprouting and sensitivity to sorafenib treatment in hepatocellular carcinoma
Authors: J Xu, J Liang, YM Meng, J Yan, XJ Yu, CQ Liu, L Xu, SM Zhuang, L Zheng
Clin. Cancer Res, 2017-02-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
DeepCAGE transcriptomics identify HOXD10 as transcription factor regulating lymphatic endothelial responses to VEGF-C
J Cell Sci, 2016-05-19;0(0):.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus.
Authors: Wang J, Tao J, Chen D, Cai J, Irani K, Wang Q, Yuan H, Chen A
Arterioscler Thromb Vasc Biol, 2013-10-31;34(0):99.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
A novel serum-free monolayer culture for orderly hematopoietic differentiation of human pluripotent cells via mesodermal progenitors.
Authors: Niwa A, Heike T, Umeda K
PLoS ONE, 2011-07-27;6(7):e22261.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Signaling hierarchy regulating human endothelial cell development.
Authors: Kelly MA, Hirschi KK
Arterioscler. Thromb. Vasc. Biol., 2009-02-12;29(5):718-24.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Prolonged culturing of iPSC-derived brain endothelial-like cells is associated with quiescence, downregulation of glycolysis, and resistance to disruption by an Alzheimer’s brain milieu
Authors: Lindsey M. Williams, Takashi Fujimoto, Riley R. Weaver, Aric F. Logsdon, Kira M. Evitts, Jessica E. Young et al.
Fluids and Barriers of the CNS
-
WASp controls oriented migration of endothelial cells to achieve functional vascular patterning
Authors: André Rosa, Wolfgang Giese, Katja Meier, Silvanus Alt, Alexandra Klaus-Bergmann, Lowell T. Edgar et al.
Development
-
PPIL4 is essential for brain angiogenesis and implicated in intracranial aneurysms in humans
Authors: T Barak, E Ristori, AG Ercan-Senc, DF Miyagishim, C Nelson-Wil, W Dong, SC Jin, A Prendergas, W Armero, O Henegariu, EZ Erson-Omay, AS Harmanc?, M Guy, B Gültekin, D Kilic, DK Rai, N Goc, SM Aguilera, B Gülez, S Altinok, K Ozcan, Y Yarman, S Coskun, E Sempou, E Deniz, J Hintzen, A Cox, E Fomchenko, SW Jung, AK Ozturk, A Louvi, K Bilgüvar, ES Connolly, MK Khokha, KT Kahle, K Yasuno, RP Lifton, K Mishra-Gor, S Nicoli, M Günel
Nature Medicine, 2021-12-09;27(12):2165-2175.
-
Genome-wide differential expression profiling of lncRNAs and mRNAs in human induced pluripotent stem cell-derived endothelial cells exposed to e-cigarette extract
Authors: Hoai Huong Thi Le, Chen-wei Liu, Philip Denaro, Jordan Jousma, Ning-Yi Shao, Irfan Rahman et al.
Stem Cell Research & Therapy
-
Motor neurons use push-pull signals to direct vascular remodeling critical for their connectivity
Authors: Luis F. Martins, Ilaria Brambilla, Alessia Motta, Stefano de Pretis, Ganesh Parameshwar Bhat, Aurora Badaloni et al.
Neuron
-
Patient-specific and gene-corrected induced pluripotent stem cell-derived endothelial cells elucidate single-cell phenotype of pulmonary veno-occlusive disease
Authors: Baihui Ma, Tianjiao Li, Wenke Li, Hang Yang, Qixian Zeng, Zihang Pan et al.
Stem Cell Reports
-
Advances in blood–brain barrier modeling in microphysiological systems highlight critical differences in opioid transport due to cortisol exposure
Authors: Jacquelyn A. Brown, Shannon L. Faley, Yajuan Shi, Kathleen M. Hillgren, Geri A. Sawada, Thomas K. Baker et al.
Fluids and Barriers of the CNS
-
YAP and the RhoC regulator ARHGAP18, are required to mediate flow-dependent endothelial cell alignment
Authors: Paul R. Coleman, Angelina J. Lay, Ka Ka Ting, Yang Zhao, Jia Li, Sorour Jarrah et al.
Cell Communication and Signaling
-
Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation
Authors: Alexandra Brahmer, Elmo Neuberger, Leona Esch-Heisser, Nils Haller, Malene Moeller Jorgensen, Rikke Baek et al.
Journal of Extracellular Vesicles
-
Endothelial EphB4 maintains vascular integrity and transport function in adult heart
Authors: G Luxán, J Stewen, N Díaz, K Kato, SK Maney, A Aravamudha, F Berkenfeld, N Nagelmann, HC Drexler, D Zeuschner, C Faber, H Schillers, S Hermann, J Wiseman, JM Vaquerizas, ME Pitulescu, RH Adams
Elife, 2019-11-29;8(0):.
-
PI3K/AKT/Afadin signaling pathway contributes to pathological vascularization in glioblastomas
Authors: Xuan Zhai, Yingliang Li, Ping Liang, Lusheng Li, Yudong Zhou, Weidan Zhang et al.
Oncology Letters
-
Murine endothelial serine palmitoyltransferase 1 (SPTLC1) is required for vascular development and systemic sphingolipid homeostasis
Authors: Kuo A, Checa A, Niaudet C et al.
eLife
-
A Fully Automated High-Throughput Bioreactor System for Lung Regeneration.
Authors: Gorman DE, Wu T, Gilpin SE, Ott HC.
Tissue Eng Part C Methods.
-
Competition for endothelial cell polarity drives vascular morphogenesis in the mouse retina
Authors: Pedro Barbacena, Maria Dominguez-Cejudo, Catarina G. Fonseca, Manuel Gómez-González, Laura M. Faure, Georgia Zarkada et al.
Developmental Cell
-
Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging
Authors: Nicholas E. Propson, Ethan R. Roy, Alexandra Litvinchuk, Jörg Köhl, Hui Zheng
Journal of Clinical Investigation
-
Cooperative ETS transcription factors enforce adult endothelial cell fate and cardiovascular homeostasis
Authors: Jesus M. Gomez-Salinero, Tomer Itkin, Sean Houghton, Chaitanya Badwe, Yang Lin, Viktoria Kalna et al.
Nature Cardiovascular Research
-
Oxidative pentose phosphate pathway controls vascular mural cell coverage by regulating extracellular matrix composition
Authors: Nicola Facchinello, Matteo Astone, Matteo Audano, Roxana E. Oberkersch, Marianna Spizzotin, Enrica Calura et al.
Nature Metabolism
-
Quantitative Label‐Free Imaging of 3D Vascular Networks Self‐Assembled in Synthetic Hydrogels
Authors: Gaurav Kaushik, Daniel A. Gil, Elizabeth Torr, Elizabeth S. Berge, Cheryl Soref, Peyton Uhl et al.
Advanced Healthcare Materials
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human VE-Cadherin Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human VE-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: