Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antibody Summary
Met1-Lys322
Accession # P05089
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Arginase 1/ARG1 in HepG2 Human Cell Line by Flow Cytometry. HepG2 human hepatocellular carcinoma cell line was stained with Sheep Anti-Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antigen Affinity-purified Polyclonal Antibody (Catalog # IC5868F, filled histogram) or isotype control antibody (Catalog # IC016F, open histogram). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Detection of Arginase 1/ARG1 in Hepa 1‑6 Mouse Cell Line by Flow Cytometry. Hepa 1-6 mouse hepatoma cell line was stained with Sheep Anti-Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antigen Affinity-purified Polyclonal Antibody (Catalog # IC5868F, filled histogram) or isotype control antibody (Catalog # IC016F, open histogram). To facilitate intracellular staining, cells were fixed with Flow Cytometry Fixation Buffer (Catalog # FC004) and permeabilized with Flow Cytometry Permeabilization/Wash Buffer I (Catalog # FC005). View our protocol for Staining Intracellular Molecules.
 View Larger
View Larger
Detection of Human Arginase 1/ARG1/liver Arginase by Flow Cytometry Within PBMCs population, GSC-derived exosomes promote an immunosuppressive phenotype in monocytes and stimulate the production of arginase-1 and IL-10 by Mo-MDSCs.Unstimulated PBMCs were incubated in absence (CTRL) or presence (GSC-EXO) of GSC-derived exosomes. Cells were surface stained with anti-CD14, anti CD33, anti CD11b and HLA-DR and then stained to detect intracellular level of IL-10 and arginase-1 by flow cytometry. (A) Gating strategy: physical parameters, i.e. forward scatter (FSC) and side scatter (SSC), were used to select monocytes (gate R3, left panel). Monocytes were recognized evaluating the expression of CD11b/CD33 (gate R4, middle panel) and CD14/HLA-DR (gate R5, right panel). (B) Representative FACS histograms of the intracellular staining of IL-10 and arginase-1 and of HLA-DR staining of CD14+/CD11b/CD33+ cells are shown. (C) The percentage of cells expressing IL-10 and arginase-1 and the MFI ratio of HLA-DR expression are shown (n = 6); bars, SD;*, significantly different from the control; P<0.05. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0169932), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human Arginase 1/ARG1/liver Arginase by Flow Cytometry Ultracentrifugated (UC) GSC-derived exosomes promote an immunosuppressive phenotype in monocytes similarly to ExoQuick (EQ) purified GSC- or GBM-derived exosomes.(A) CFSE-labelled PBMCs isolated from healthy donors (left) or CD14 negatively-sorted PBMCs (CD14-) (right) were pre-treated for 24 hours without (white column, CTRL) or with EQ or UC isolated GSC-derived exosomes (black column, EQ GSC-EXO; grey column, UC GSC-EXO, respectively) and stimulated for 4 days with anti-CD3 and anti-CD28. Histograms show a significant difference in the percentage of proliferating CD3+ (n = 4); bars, SD;*; from the control; P<0.05. (B-D) Induction of a Mo-MDSC phenotype on monocytes. Unstimulated PBMCs were incubated in the absence (CTRL) or presence of GSC-derived exosomes purified by EQ (black column, EQ EXO-GSC) or UC(grey column, UC EXO-GSC). Cells were surface stained with (B) anti-CD14, anti CD33, anti CD11b and (C) HLA-DR and then stained to detect intracellular level of IL-10 and arginase-1 by flow cytometry. (B) Gating strategy: physical parameters, i.e. forward scatter (FSC) and side scatter (SSC), were used to select monocytes (gate R1, left panel). Monocytes were recognized by evaluating the expression of CD11b/CD33 (gate R2, middle panel) and CD14/HLA-DR (gate R3, right panel). (C) Representative FACS histograms of the intracellular staining of IL-10 and arginase-1 and of HLA-DR staining of CD14+/CD11b/CD33+ cells are shown. (D) The percentage of cells expressing IL-10 and arginase-1 and the MFI ratio of HLA-DR expression are shown (n = 3); bars, SD;*, significantly different from the control; *, P<0.05; **,P<0.01. (E) PBMCs were stimulated with anti-CD3 and anti-CD28 in the absence (white column, CTRL) or presence (black column, GBM-EXO) of exosomes isolated from plasma of glioblastoma patients (GBM) by either EQ or UC and used at 1:10 dilution. Healthy donor plasma-derived exosomes were used as a control (striped column, EXO-HEALTHY). Proliferation of CD3+ was measured by CFSE assay at day 4. Proliferation in the presence of exosomes was normalized to CD3+ cell proliferation in the absence of exosomes (CTRL set to 100%). (F) Proliferation of both unfractioned PBMCs and PBMCs depleted of the CD14+ population (CD14-) was measured, after CFSE labelling assay, by flow cytometry. Cells were pre-incubated without (white column, CTRL) or with EQGBM-derived exosomes (black column, EQ GBM-EXO) or UC GBM-derived exosomes (grey column, UC GBM-EXO). Columns, mean (n = 4); bars, SD; *, significantly different from the control; P<0.05. Image collected and cropped by CiteAb from the following publication (https://dx.plos.org/10.1371/journal.pone.0169932), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
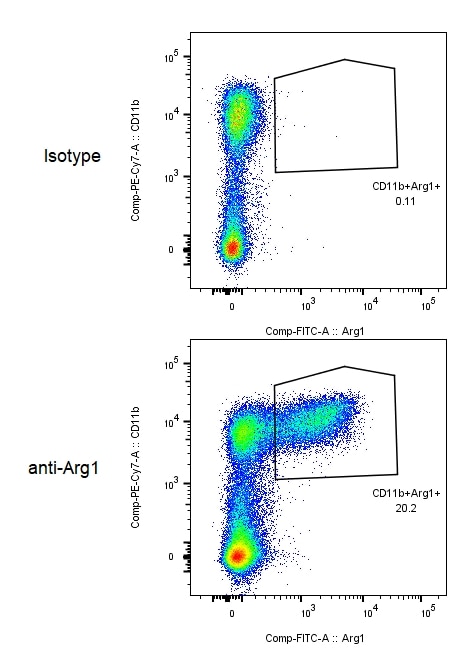
Detection of Human Arginase 1/ARG1/liver Arginase by Flow Cytometry Arginase 1 expression in different subsets of circulating MDSC in patients with PC. Flow cytometric evaluation of ARG1 expression in CD33, CD11b, CD15 and CD14 in whole blood is shown in the representative dot plots. Gates were set based on negative controls. Numbers represent the percentages from the original populations gated. P (number) above each FACS plot indicates the population gated which was analysed. The gate was first set on HLA-DR negative against side scatter as shown in the top dot plot. Next, the CD11b+ & CD33+ (right) and CD14+ & CD15+ (left) subsets were identified. The expression of ARG1 in each subset was then determined as shown in the bottom dot plots. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/24741628), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, 2 to 8 °C as supplied.
Background: Arginase 1/ARG1
Arginase 1 (ARG1) is a 35‑40 kDa member of the arginase family of enzymes. It is expressed in multiple cell types, including erythrocytes, hepatocytes, neutrophils, smooth muscle and macrophages. ARG1 demonstrates two distinct functions: in the hepatocyte cytoplasm, it catalyzes the conversion of arginine to ornithine and urea, while in multiple cells, it degrades arginine, thus indirectly downregulating NO synthase (NOS) activity by depriving this enzyme of its substrate. Human AGR1 is 322 amino acids (aa) in length. Its enzyme region comprises aa 9‑309 and contains two Mn atoms. ARG1 is modestly active as a monomer, but highly active as a 105 kDa homotrimer. Trimerization is promoted by nitrosylation of Cys303, creating a regulatory feedback loop with NOS. There are two isoform variants, one that shows an eight aa insertion after Gln43, and another that shows a deletion of aa 204‑289. Full-length human ARG1 shares 87% aa identity with mouse and rat ARG1.
Product Datasheets
Citations for Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
33
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
The common parasite Toxoplasma gondii induces prostatic inflammation and microglandular hyperplasia in a mouse model.
Authors: Colinot DL, Garbuz T, Bosland MC et al.
Prostate
-
Macrophages Expressing GALC Improve Peripheral Krabbe Disease by a Mechanism Independent of Cross-Correction
Authors: Weinstock NI, Shin D, Dhimal N et al.
Neuron
-
The pro-tumoral and anti-tumoral roles of EphA4 on T regulatory cells and tumor associated macrophages during HNSCC tumor progression
Authors: Corbo, S;Nguyen, D;Bhatia, S;Darragh, LB;Abdelazeem, KNM;Court, BV;Olimpo, NA;Gadwa, J;Yu, J;Hodgson, C;Samedi, V;Garcia, ES;Siu, L;Saviola, A;Heasley, LE;Knitz, MW;Pasquale, EB;Karam, SD;
bioRxiv : the preprint server for biology
Species: Human
Sample Types:
Applications: Flow Cytometry -
EMT activates exocytotic Rabs to coordinate invasion and immunosuppression in lung cancer
Authors: Xiao GY, Tan X, Rodriguez BL et al.
Proceedings of the National Academy of Sciences of the United States of America
-
Extrinsic KRAS Signaling Shapes the Pancreatic Microenvironment Through Fibroblast Reprogramming
Authors: Ashley Velez-Delgado, Katelyn L. Donahue, Kristee L. Brown, Wenting Du, Valerie Irizarry-Negron, Rosa E. Menjivar et al.
Cellular and Molecular Gastroenterology and Hepatology
-
Dysregulated Microglial Cell Activation and Proliferation Following Repeated Antigen Stimulation
Authors: S Prasad, WS Sheng, S Hu, P Chauhan, JR Lokensgard
Frontiers in Cellular Neuroscience, 2021-08-10;15(0):686340.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Pneumococcal Extracellular Vesicles Modulate Host Immunity
Authors: SS Yerneni, S Werner, JH Azambuja, N Ludwig, R Eutsey, SD Aggarwal, PC Lucas, N Bailey, TL Whiteside, PG Campbell, NL Hiller
MBio, 2021-07-13;0(0):e0165721.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
p53 loss activates prometastatic secretory vesicle biogenesis in the Golgi
Authors: X Tan, P Banerjee, L Shi, GY Xiao, BL Rodriguez, CL Grzeskowia, X Liu, J Yu, DL Gibbons, WK Russell, CJ Creighton, JM Kurie
Science Advances, 2021-06-18;7(25):.
Species: Mouse
Sample Types: Tissue Homogenates
Applications: ICC -
Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon
Authors: A Nakamura, S Kurihara, D Takahashi, W Ohashi, Y Nakamura, S Kimura, M Onuki, A Kume, Y Sasazawa, Y Furusawa, Y Obata, S Fukuda, S Saiki, M Matsumoto, K Hase
Nature Communications, 2021-04-08;12(1):2105.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy
Authors: A Rodriguez-, RC Lynn, M Poussin, MA Eiva, LC Shaw, RS O'Connor, NG Minutolo, V Casado-Med, G Lopez, T Matsuyama, DJ Powell
Nature Communications, 2021-02-09;12(1):877.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Nod1 promotes colorectal carcinogenesis by regulating the immunosuppressive functions of tumor-infiltrating myeloid cells
Authors: C Maisonneuv, DKL Tsang, EG Foerster, LM Robert, T Mukherjee, D Prescott, I Tattoli, P Lemire, DA Winer, S Winer, CJ Streutker, K Geddes, K Cadwell, RL Ferrero, A Martin, SE Girardin, DJ Philpott
Cell Reports, 2021-01-26;34(4):108677.
Species: Mouse, Transgenic Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Tumor-derived exosomes promote angiogenesis via adenosine A2B receptor signalling
Authors: Nils Ludwig, Saigopalakrishna S. Yerneni, Juliana H. Azambuja, Delbert G. Gillespie, Elizabeth V. Menshikova, Edwin K. Jackson et al.
Angiogenesis
-
Myeloid-derived suppressor cell depletion therapy targets IL-17A-expressing mammary carcinomas
Authors: B Dawod, J Liu, S Gebremeske, C Yan, A Sappong, B Johnston, DW Hoskin, JS Marshall, J Wang
Sci Rep, 2020-08-07;10(1):13343.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes
Authors: N Gomez-Lope, M Arenas-Her, R Romero, D Miller, V Garcia-Flo, Y Leng, Y Xu, J Galaz, SS Hassan, CD Hsu, H Tse, C Sanchez-To, B Done, AL Tarca
Cell Rep, 2020-07-07;32(1):107874.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Hippocampal neural stem cells and microglia response to experimental inflammatory bowel disease (IBD)
Authors: IA Gampieraki, Y Koutmani, M Semitekolo, I Morianos, I Charalampo, G Xanthou, A Gravanis, KP Karalis
Mol. Psychiatry, 2020-01-22;0(0):.
Species: Mouse
Sample Types: Tissue
Applications: Flow Cytometry -
beta 2 adrenergic receptor–mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells
Authors: Hemn Mohammadpour, Cameron R. MacDonald, Guanxi Qiao, Minhui Chen, Bowen Dong, Bonnie L. Hylander et al.
Journal of Clinical Investigation
-
Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8+ T Cell-Derived Interferon-gamma
Authors: Chang Liu, Maria Chikina, Rahul Deshpande, Ashley V. Menk, Ting Wang, Tracy Tabib et al.
Immunity
-
Astragalus Polysaccharide RAP Induces Macrophage Phenotype Polarization to M1 via the Notch Signaling Pathway
Authors: W Wei, ZP Li, ZX Bian, QB Han
Molecules, 2019-05-27;24(10):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue
Authors: Hui Zhao, Qianwen Shang, Zhenzhen Pan, Yang Bai, Zequn Li, Huiying Zhang et al.
Diabetes
-
Phosphorylated exogenous alpha-synuclein fibrils exacerbate pathology and induce neuronal dysfunction in mice
Authors: M Karampetso, MT Ardah, M Semitekolo, A Polissidis, M Samiotaki, M Kalomoiri, N Majbour, G Xanthou, OMA El-Agnaf, K Vekrellis
Sci Rep, 2017-11-28;7(1):16533.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival
Authors: Konrad Buscher, Erik Ehinger, Pritha Gupta, Akula Bala Pramod, Dennis Wolf, George Tweet et al.
Nature Communications
-
NK Cells Alleviate Lung Inflammation by Negatively Regulating Group 2 Innate Lymphoid Cells
Authors: J Bi, L Cui, G Yu, X Yang, Y Chen, X Wan
J. Immunol, 2017-03-08;0(0):.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Modulation of Microglial Cell Fc? Receptor Expression Following Viral Brain Infection
Authors: P Chauhan, S Hu, WS Sheng, S Prasad, JR Lokensgard
Sci Rep, 2017-02-06;7(0):41889.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells
Authors: R Domenis, D Cesselli, B Toffoletto, E Bourkoula, F Caponnetto, I Manini, AP Beltrami, T Ius, M Skrap, C Di Loreto, G Gri
PLoS ONE, 2017-01-20;12(1):e0169932.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
Blockade of MK2 is protective in inflammation-associated colorectal cancer development
Authors: Anita L. Ray, Eliseo F. Castillo, Katherine T. Morris, Robert A. Nofchissey, Lea L. Weston, Von G. Samedi et al.
International Journal of Cancer
-
Targeting Ornithine Decarboxylase by ?-Difluoromethylornithine Inhibits Tumor Growth by Impairing Myeloid-Derived Suppressor Cells.
Authors: Cong Ye, Zhe Geng, Donye Dominguez, Siqi Chen, Jie Fan, Lei Qin, Alan Long, Yi Zhang, Timothy M Kuzel, Bin Zhang
Journal of Immunology (Baltimore, Md. : 1950), 2015-12-09;0(0):1550-6606.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients.
Authors: Khaled, Yazan S, Ammori, Basil J, Elkord, Eyad
J Immunol Res, 2014-01-29;2014(0):879897.
Species: Human
Sample Types: Whole Blood
Applications: Flow Cytometry -
Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection.
Authors: Elahi, Shokroll, Ertelt, James M, Kinder, Jeremy M, Jiang, Tony T, Zhang, Xuzhe, Xin, Lijun, Chaturvedi, Vandana, Strong, Beverly, Qualls, Joseph E, Steinbrecher, Kris A, Kalfa, Theodosi, Shaaban, Aimen F, Way, Sing Sin
Nature, 2013-11-06;504(7478):158-62.
Species: Mouse
Sample Types: Whole Cells
Applications: Flow Cytometry -
Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples.
J. Immunol. Methods, 2012-04-13;381(1):14-22.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
STING signaling remodels the tumor microenvironment by antagonizing myeloid-derived suppressor cell expansion
Authors: CX Zhang, SB Ye, JJ Ni, TT Cai, YN Liu, DJ Huang, HQ Mai, QY Chen, J He, XS Zhang, YX Zeng, J Li, J Cui
Cell Death Differ., 2019-02-28;0(0):.
-
Location of tumor affects local and distant immune cell type and number
Authors: JA Hensel, V Khattar, R Ashton, C Lee, GP Siegal, S Ponnazhaga
Immun Inflamm Dis, 2017-02-23;5(1):85-94.
-
Myeloid cell plasticity in the evolution of central nervous system autoimmunity.
Authors: Giles DA, Washnock-Schmid JM, Duncker PC et al.
Ann. Neurol.
-
STAT3 Inhibition Combined with CpG Immunostimulation Activates Antitumor Immunity to Eradicate Genetically Distinct Castration-Resistant Prostate Cancers.
Authors: Moreira D, Adamus T, Zhao X et al.
Clin. Cancer Res.
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antibody
Average Rating: 5 (Based on 1 Review)
Have you used Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by: