Human IL-29/IL-28B (IFN-lambda 1/3) DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-29/IL-28B (IFN-lambda 1/3). The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
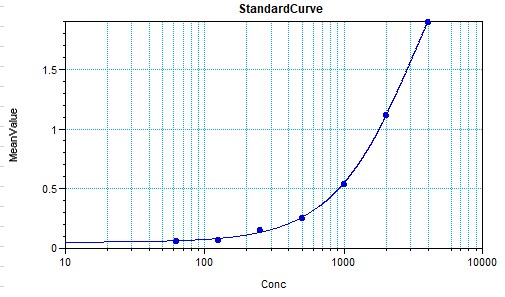
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-29/IL-28B (IFN-lambda 1/3)
Human/mouse IL-28A (IFN-lambda 2), IL-28B (IFN-lambda 3), and human IL-29 (IFN-lambda 1) are class II cytokine receptor ligands that are distantly related to members of the IL-10 family (11 - 13% amino acid (aa) sequence identity) and type I IFN family (15 - 19% aa sequence identity). These cytokines exert bioactivities that overlap those of type I IFNs, including antiviral activity and up-regulation of MHC class I antigen expression. The proteins signal through the same heterodimeric receptor complex that is composed of the IL-10 receptor beta (IL-10 R beta) and a novel IL-28 receptor alpha (IL-28 R alpha, also known as IFN-lambda R1). Mouse IL-28 shares 61%, 62%, and 52% aa identity with human IL-28A, IL-28B, and IL-29, respectively.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-29/IL-28B (IFN-lambda 1/3) DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
32
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants
Authors: Reuschl, AK;Thorne, LG;Whelan, MVX;Ragazzini, R;Furnon, W;Cowton, VM;De Lorenzo, G;Mesner, D;Turner, JLE;Dowgier, G;Bogoda, N;Bonfanti, P;Palmarini, M;Patel, AH;Jolly, C;Towers, GJ;
Nature microbiology
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-?3 is induced by Leishmania donovani and can inhibit parasite growth in cell line models but not in the mouse model, while it shows a significant association with leishmaniasis in humans
Authors: De, M;Sukla, S;Bharatiya, S;Keshri, S;Roy, DG;Roy, S;Dutta, D;Saha, S;Ejazi, SA;Ravichandiran, V;Ali, N;Chatterjee, M;Chinnaswamy, S;
Infection and immunity
Species: Human
Sample Types: Cell Culture Supernates
-
ZIKV induction of tristetraprolin in endothelial and Sertoli cells post-transcriptionally inhibits IFN?/? expression and promotes ZIKV persistence
Authors: Schutt, WR;Conde, JN;Mladinich, MC;Himmler, GE;Mackow, ER;
mBio
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of novel lactic acid bacteria with enhanced protective effects against influenza virus
Authors: Sugimoto, A;Numaguchi, T;Chihama, R;Takenaka, Y;Sato, Y;
PloS one
Species: Human
Sample Types: Cell Culture Supernates
-
Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1
Authors: H Stölting, L Baillon, R Frise, K Bonner, RJ Hewitt, PL Molyneaux, ML Gore, Breathing, WS Barclay, S Saglani, CM Lloyd
Mucosal Immunology, 2022-07-15;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Deficiency in coatomer complex I causes aberrant activation of STING signalling
Authors: A Steiner, K Hrovat-Sch, I Prigione, CH Yu, P Laohamonth, CR Harapas, RRJ Low, D De Nardo, LF Dagley, MJ Mlodzianos, KL Rogers, T Zillinger, G Hartmann, MP Gantier, M Gattorno, M Geyer, S Volpi, S Davidson, SL Masters
Nature Communications, 2022-04-28;13(1):2321.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibitor of growth protein 3 epigenetically silences endogenous retroviral elements and prevents innate immune activation
Authors: Y Song, G Hou, J Diep, YS Ooi, NS Akopyants, SM Beverley, JE Carette, HB Greenberg, S Ding
Nucleic Acids Research, 2021-12-16;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2 Nucleocapsid Protein Targets RIG-I-Like Receptor Pathways to Inhibit the Induction of Interferon Response
Authors: SJ Oh, OS Shin
Cells, 2021-03-02;10(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
Extracellular vesicles promote transkingdom nutrient transfer during viral-bacterial co-infection
Authors: MR Hendricks, S Lane, JA Melvin, Y Ouyang, DB Stolz, JV Williams, Y Sadovsky, JM Bomberger
Cell Reports, 2021-01-26;34(4):108672.
Species: Human
Sample Types: Cell Culture Supernates
-
The regulation and pharmacological modulation of immune complex induced type III IFN production by plasmacytoid dendritic cells
Authors: K Hjorton, N Hagberg, P Pucholt, ML Eloranta, L Rönnblom
Arthritis Res. Ther., 2020-06-05;22(1):130.
Species: Human
Sample Types: Cell Culture Supernates
-
Dysbiotic oral microbiota and infected salivary glands in Sj�gren's syndrome
Authors: J Alam, A Lee, J Lee, DI Kwon, HK Park, JH Park, S Jeon, K Baek, J Lee, SH Park, Y Choi
PLoS ONE, 2020-03-24;15(3):e0230667.
Species: Human
Sample Types: Cell Culture Supernates
-
MicroRNA-122 supports robust innate immunity in hepatocytes by targeting the RTKs/STAT3 signaling pathway
Authors: H Xu, SJ Xu, SJ Xie, Y Zhang, JH Yang, WQ Zhang, MN Zheng, H Zhou, LH Qu
Elife, 2019-02-08;8(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-17A Attenuates IFN-? Expression by Inducing Suppressor of Cytokine Signaling Expression in Airway Epithelium
Authors: M Niwa, T Fujisawa, K Mori, K Yamanaka, H Yasui, Y Suzuki, M Karayama, H Hozumi, K Furuhashi, N Enomoto, Y Nakamura, N Inui, T Suzuki, M Maekawa, T Suda
J. Immunol., 2018-09-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
ZIKV Infection Induces an Inflammatory Response but Fails to Activate Types I, II, and III IFN Response in Human PBMC
Authors: F Colavita, V Bordoni, C Caglioti, M Biava, C Castillett, L Bordi, S Quartu, M Iannetta, G Ippolito, C Agrati, MR Capobianch, E Lalle
Mediators Inflamm., 2018-06-03;2018(0):2450540.
Species: Human
Sample Types: Cell Culture Supernates
-
STAG2 deficiency induces interferon responses via cGAS-STING pathway and restricts virus infection
Authors: S Ding, J Diep, N Feng, L Ren, B Li, YS Ooi, X Wang, KF Brulois, LL Yasukawa, X Li, CJ Kuo, DA Solomon, JE Carette, HB Greenberg
Nat Commun, 2018-04-16;9(1):1485.
Species: Human
Sample Types: Cell Culture Supernates
-
PU.1 acts as tumor suppressor for myeloma cells through direct transcriptional repression of IRF4
Authors: N Ueno, N Nishimura, S Ueno, S Endo, H Tatetsu, S Hirata, H Hata, M Matsuoka, H Mitsuya, Y Okuno
Oncogene, 2017-04-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Mast cells are permissive for rhinovirus replication: potential implications for asthma exacerbations
Authors: Charlene Akoto
Clin. Exp. Allergy, 2017-01-26;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro
Authors: Luminita A Stanciu
Antiviral Res., 2016-11-10;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-Alpha receptor-1 upregulation in PBMC from HCV naive patients carrying cc genotype. possible role of IFN-lambda.
Authors: Lalle E, Bordi L, Caglioti C, Garbuglia A, Castilletti C, Taibi C, Cristofari F, Capobianchi M
PLoS ONE, 2014-04-01;9(4):e93434.
Species: Human
Sample Types: Cell Culture Supernates
-
Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells.
Authors: Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S, Szabo G
PLoS ONE, 2012-10-10;7(10):e44915.
Species: Human
Sample Types: Serum
-
Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells.
Authors: Yin Z, Dai J, Deng J, Sheikh F, Natalia M, Shih T, Lewis-Antes A, Amrute S, Garrigues U, Doyle S, Donnelly R, Kotenko S, Fitzgerald-Bocarsly P
J Immunol, 2012-08-13;189(6):2735-45.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta.
Authors: Bierne H, Travier L, Mahlakoiv T, Tailleux L, Subtil A, Lebreton A, Paliwal A, Gicquel B, Staeheli P, Lecuit M, Cossart P
PLoS ONE, 2012-06-14;7(6):e39080.
Species: Human
Sample Types: Cell Culture Supernates
-
Dobrava-Belgrade hantavirus from Germany shows receptor usage and innate immunity induction consistent with the pathogenicity of the virus in humans.
Authors: Popugaeva E, Witkowski PT, Schlegel M, Ulrich RG, Auste B, Rang A, Kruger DH, Klempa B
PLoS ONE, 2012-04-24;7(4):e35587.
Species: Primate
Sample Types: Cell Culture Supernates
-
Measles virus causes immunogenic cell death in human melanoma.
Authors: Donnelly, O G, Errington-Mais, F, Steele, L, Hadac, E, Jennings, V, Scott, K, Peach, H, Phillips, R M, Bond, J, Pandha, H, Harrington, K, Vile, R, Russell, S, Selby, P, Melcher, A A
Gene Ther, 2011-12-15;20(1):7-15.
Species: Human
Sample Types: Cell Culture Supernates
-
Herpes simplex encephalitis in children with autosomal recessive and dominant TRIF deficiency.
Authors: Sancho-Shimizu V, Perez de Diego R, Lorenzo L, Halwani R, Alangari A, Israelsson E, Fabrega S, Cardon A, Maluenda J, Tatematsu M, Mahvelati F, Herman M, Ciancanelli M, Guo Y, AlSum Z, Alkhamis N, Al-Makadma AS, Ghadiri A, Boucherit S, Plancoulaine S, Picard C, Rozenberg F, Tardieu M, Lebon P, Jouanguy E, Rezaei N, Seya T, Matsumoto M, Chaussabel D, Puel A, Zhang SY, Abel L, Al-Muhsen S, Casanova JL
J. Clin. Invest., 2011-11-21;121(12):4889-902.
Species: Human
Sample Types: Cell Culture Supernates
-
Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFN-lambda) in cutaneous lupus erythematosus.
Authors: Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, Wenzel J
J. Invest. Dermatol., 2010-08-19;131(1):133-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Inhibition of dynamin-dependent endocytosis interferes with type III IFN expression in bacteria-infected human monocyte-derived DCs.
Authors: Pietila TE, Latvala S, Osterlund P, Julkunen I
J. Leukoc. Biol., 2010-07-07;88(4):665-74.
Species: Human
Sample Types: Cell Culture Supernates
-
Alpha/beta interferon (IFN-alpha/beta)-independent induction of IFN-lambda1 (interleukin-29) in response to Hantaan virus infection.
Authors: Stoltz M, Klingstrom J
J. Virol., 2010-06-30;84(18):9140-8.
Species: Human
Sample Types: Cell Culture Supernates
-
IRF5 is required for late-phase TNF secretion by human dendritic cells.
Authors: Krausgruber T, Saliba D, Ryzhakov G
Blood, 2010-03-17;115(22):4421-30.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-4 enhances IFN-lambda1 (IL-29) production by plasmacytoid DCs via monocyte secretion of IL-1Ra.
Authors: Megjugorac NJ, Gallagher GE, Gallagher G
Blood, 2010-03-16;115(21):4185-90.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses.
Authors: Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB
J. Immunol., 2009-11-04;183(11):6989-97.
Species: Human
Sample Types: Cell Culture Supernates
-
Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes.
Authors: Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, Thilo J, Asadullah K, Sterry W, Volk HD, Sabat R
J. Leukoc. Biol., 2008-02-15;83(5):1181-93.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-29/IL-28B (IFN-lambda 1/3) DuoSet ELISA
Average Rating: 4.3 (Based on 4 Reviews)
Have you used Human IL-29/IL-28B (IFN-lambda 1/3) DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
This ELISA is good as there are still few reagents for studying type III interferons. I have used it many times, successfully. My only concern is the quality of the standard. Although I aliquot the standard and store it at -80, but I can see obvious decreases in my standard curve over time. I have also used the DuoSet IFNB ELISA and I do not observe these decreases in the quality of the standard