Human IL-2 Quantikine ELISA Kit Summary
Product Summary
Recovery
| Sample Type | Average % Recovery | Range % |
|---|---|---|
| Cell Culture Media (n=5) | 101 | 96-107 |
| Citrate Plasma (n=5) | 105 | 98-111 |
| EDTA Plasma (n=5) | 118 | 104-139 |
| Heparin Plasma (n=5) | 109 | 94-129 |
| Serum (n=5) | 99 | 89-114 |
Linearity
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-2
Interleukin 2 (IL-2), also known as T cell growth factor (TCGF), is a 15-18 kDa variably glycosylated alpha -helical polypeptide that is a member of the Common gamma Chain ( gamma c) cytokine family (1-4). It exists as a monomer and has a notably short half-life (< 30 minutes) (1). Human IL-2 is synthesized as a 153 amino acid (aa) precursor that contains a 20 aa signal sequence plus a 133 aa mature region (5, 6). The mature region is alpha -helical in nature, and contains one utilized O-linked glycosylation site at Thr3 plus three cysteines, two of which form an intrachain disulfide bond that is essential for activity (7). Mature human IL-2 shares 73%, 66%, 78% and 97% aa identity with canine, rat, feline and rhesus monkey IL-2, respectively. Although human IL-2 shares only approximately 60% aa identity with the highly polymorphic mouse IL-2, human IL-2 is known to be active on mouse IL-2 responsive cells. Cells reported to secrete IL-2 include gamma δ T cells (8), activated conventional CD4+ and CD8+ T cells (1, 9), neurons (10, 11), microglia (12), and hematopoietic stem cells (13).
Assay Procedure
Refer to the product- Prepare all reagents, standard dilutions, and samples as directed in the product insert.
- Remove excess microplate strips from the plate frame, return them to the foil pouch containing the desiccant pack, and reseal.
- Add 100 µL of Assay Diluent to each well.
- Add 100 µL of Standard, control, or sample to each well. Cover with a plate sealer, and incubate at room temperature for 2 hours.
- Aspirate each well and wash, repeating the process twice for a total of 3 washes.
- Add 200 µL of Conjugate to each well. Cover with a new plate sealer, and incubate at room temperature for 2 hours.
- Aspirate and wash 3 times.
- Add 200 µL Substrate Solution to each well. Incubate at room temperature for 30 minutes. PROTECT FROM LIGHT.
- Add 50 µL of Stop Solution to each well. Read at 450 nm within 30 minutes. Set wavelength correction to 540 nm or 570 nm.





Citations for Human IL-2 Quantikine ELISA Kit
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
92
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Influence of blood trace elements on immune responses and adverse symptoms subsequent to Sinopharm COVID-19 vaccination
Authors: Abbasifard, M;Kazerooni, K;Taghipour Khaje Sharifi, G;Bagheri-Hosseinabadi, Z;Hajizadeh, MR;
Scientific reports
Species: Human
Sample Types: Cell Culture Supernates
-
A Novel Monoclonal Antibody against PD-1 for the Treatment of Viral Oncogene-Induced Tumors or Other Cancer
Authors: Xu, X;Yan, SL;Yo, YT;Chiang, P;Tsai, CY;Lin, LL;Qin, A;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Ex vivo T cell differentiation in adoptive immunotherapy manufacturing: Critical process parameters and analytical technologies
Authors: Chen, S;Nguyen, TD;Lee, KZ;Liu, D;
Biotechnology advances
Species: Human
Sample Types: Cell Culture Supernates
-
A bispecific antibody that targets the membrane-proximal region of mesothelin and retains high anticancer activity in the presence of shed mesothelin
Authors: Chakraborty, A;Onda, M;O'Shea, T;Wei, J;Liu, X;Bera, TK;Pastan, I;
Molecular cancer therapeutics
Species: Human
Sample Types: Cell Culture Supernates
-
Serum Dehydroepiandrosterone sulfate (DHEA-S) level and its potential impact on immune responses and symptom severity after Oxford-AstraZeneca COVID-19 vaccination
Authors: Abbasifard, M;Dehghan Banadaki, M;Taghipour Khaje Sharifi, G;Rahnama, A;Bagheri-Hosseinabadi, Z;
International immunopharmacology
Species: Human
Sample Types: Serum
-
In the Search for Biomarkers of Pulmonary Arterial Hypertension, Are Cytokines IL-2, IL-4, IL-6, IL-10, and IFN-Gamma the Right Indicators to Use?
Authors: Tomaszewski, M;Mertowska, P;Janczewska, M;Stycze?, A;Mertowski, S;Jonas, K;Grywalska, E;Kope?, G;
International journal of molecular sciences
Species: Human
Sample Types: Plasma
-
Possible Correlation between Urocortin 1 (Ucn1) and Immune Parameters in Patients with Endometriosis
Authors: Abramiuk, M;Frankowska, K;Ku?ak, K;Tarkowski, R;Mertowska, P;Mertowski, S;Grywalska, E;
International journal of molecular sciences
Species: Human
Sample Types: Serum
-
Novel scFv against Notch Ligand JAG1 Suitable for Development of Cell Therapies toward JAG1-Positive Tumors
Authors: G Silva, AF Rodrigues, S Ferreira, C Matos, RP Eleutério, G Marques, K Kucheryava, AR Lemos, PMF Sousa, R Castro, A Barbas, D Simão, PM Alves
Biomolecules, 2023-03-02;13(3):.
Species: Human
Sample Types: Cell Culture Supernates
-
MET-induced CD73 restrains STING-mediated immunogenicity of EGFR-mutant lung cancer
Authors: R Yoshida, M Saigi, T Tani, BF Springer, H Shibata, S Kitajima, NR Mahadevan, M Campisi, W Kim, Y Kobayashi, TC Thai, K Haratani, Y Yamamoto, SK Sundararam, EH Knelson, A Vajdi, I Canadas, R Uppaluri, CP Paweletz, JJ Miret, PH Lizotte, PC Gokhale, PA Janne, DA Barbie
Cancer Research, 2022-11-02;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Development of Highly Effective Anti-Mesothelin hYP218 Chimeric Antigen Receptor T Cells With Increased Tumor Infiltration and Persistence for Treating Solid Tumors
Authors: S Tomar, J Zhang, M Khanal, J Hong, A Venugopala, Q Jiang, M Sengupta, M Miettinen, N Li, I Pastan, M Ho, R Hassan
Molecular Cancer Therapeutics, 2022-07-05;21(7):1195-1206.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Histone Methyltransferase SETDB1 Promotes Immune Evasion in Colorectal Cancer via FOSB-Mediated Downregulation of MicroRNA-22 through BATF3/PD-L1 Pathway
Authors: J Tian, W Wang, J Zhu, Y Zhuang, C Qi, Z Cai, W Yan, W Lu, A Shang
Journal of Immunology Research, 2022-04-20;2022(0):4012920.
Species: Human
Sample Types: Cell Culture Supernates
-
Switchable assembly and function of antibody complexes in�vivo using a small molecule
Authors: AJ Martinko, EF Simonds, S Prasad, A Ponce, CJ Bracken, J Wei, YH Wang, TL Chow, Z Huang, MJ Evans, JA Wells, ZB Hill
Proceedings of the National Academy of Sciences of the United States of America, 2022-03-01;119(9):.
Species: Human
Sample Types: Plasma
-
Humoral Immunity to Allogeneic Immunoproteasome-Expressing Mesenchymal Stromal Cells Requires Efferocytosis by Endogenous Phagocytes
Authors: JP Bikorimana, J Abusarah, N Salame, N El-Hachem, R Shammaa, M Rafei
Cells, 2022-02-09;11(4):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
LncRNA NEAT1 is upregulated in recurrent aphthous stomatitis (RAS) and has predictive values
Authors: Y Han, L Wang, Q Li, H Chen, X Ma
BMC Oral Health, 2021-12-31;21(1):673.
Species: Human
Sample Types: Plasma
-
Phenotypic characterization of NKT-like cells and evaluation of specifically related cytokines for the prediction of unexplained recurrent miscarriage
Authors: WS Khalaf, MRA Mahmoud, WF Elkhatib, HR Hashem, WE Soliman
Heliyon, 2021-11-16;7(11):e08409.
Species: Human
Sample Types: Serum
-
Impaired Innate Immunity in Pediatric Patients Type 1 Diabetes-Focus on Toll-like Receptors Expression
Authors: K Kurianowic, M Klatka, A Polak, A Hymos, D B?bnowska, M Podgajna, R Hrynkiewic, O Sierawska, P Nied?wiedz
International Journal of Molecular Sciences, 2021-11-09;22(22):.
Species: Human
Sample Types: Plasma
-
Topical Delivery of Rapamycin by Means of Microenvironment-Sensitive Core-Multi-Shell Nanocarriers: Assessment of Anti-Inflammatory Activity in an ex vivo Skin/T Cell Co-Culture Model
Authors: F Rancan, X Guo, K Rajes, P Sidiropoul, F Zabihi, L Hoffmann, S Hadam, U Blume-Peyt, E Rühl, R Haag, A Vogt
International Journal of Nanomedicine, 2021-10-22;16(0):7137-7151.
Species: Human
Sample Types: Cell Culture Supernates
-
Paracrine activity of adipose derived stem cells on limbal epithelial stem cells
Authors: B Sikora, A Skubis-Sik, A Prusek, J Gola
Scientific Reports, 2021-10-07;11(1):19956.
Species: Human
Sample Types: Cell Culture Supernates
-
Determination of phenolic profiles of Herniaria polygama and Herniaria incana fractions and their in vitro antioxidant and anti-inflammatory effects
Authors: J Kolodziejc, S Kozachok, ? Pecio, S Marchyshyn, W Oleszek
Phytochemistry, 2021-07-27;190(0):112861.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of Ingesting Fucoidan Derived from Cladosiphon okamuranus Tokida on Human NK Cells: A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Pilot Study
Authors: M Tomori, T Nagamine, T Miyamoto, M Iha
Marine Drugs, 2021-06-15;19(6):.
Species: Human
Sample Types: Plasma
-
Pomalidomide restores immune recognition of primary effusion lymphoma through upregulation of ICAM-1 and B7-2
Authors: P Shrestha, DA Davis, HK Jaeger, A Stream, AI Aisabor, R Yarchoan
PloS Pathogens, 2021-01-07;17(1):e1009091.
Species: Human
Sample Types: Cell Culture Supernates
-
Synthesis and Characterization of Store-Operated Calcium Entry Inhibitors Active in the Submicromolar Range
Authors: C Le Guilche, T Luyten, JB Parys, M Pucheault, O Dellis
International Journal of Molecular Sciences, 2020-12-21;21(24):.
Species: Human
Sample Types: Cell Culture Supernates
-
Aberrant recruitment of leukocytes defines poor wound healing in patients with recessive dystrophic epidermolysis bullosa
Authors: T Phillips, L Huitema, R Cepeda, DL Cobos, RIM Perez, MS Garza, F Ringpfeil, B Dasgeb, J Uitto, JC Salas-Alan, V Alexeev, O Igoucheva
J Dermatol Sci, 2020-10-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
'Psoriasis 1' reduces T‑lymphocyte‑mediated inflammation in patients with psoriasis by inhibiting vitamin D receptor‑mediated STAT4 inactivation.
Authors: Gao Y, Sun W, Cha X, Wang H
Int J Mol Med, 2020-08-06;46(4):1538-1550.
Species: Human
Sample Types: Cell Culture Supernates
-
Identification of Novel Molecular Markers of Human Th17 Cells
Authors: A Sa?kowska, K Kara?, I Karwaciak, A Walczak-Dr, M Krawczyk, M Sobalska-K, J Dastych, M Ratajewski
Cells, 2020-07-03;9(7):.
Species: Human
Sample Types: Cell Culture Supernates
-
Overexpression of PD-1 on Peripheral Blood Lymphocytes in Patients with Idiopathic Pulmonary Arterial Hypertension and Its Association with High Viral Loads of Epstein-Barr Virus and Poor Clinical Parameters
Authors: M Tomaszewsk, E Grywalska, A Tomaszewsk, P B?aszczak, M Kurzyna, J Roli?ski, G Kope?
J Clin Med, 2020-06-24;9(6):.
Species: Human
Sample Types: Plasma
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Plasma
-
CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL?2 and TGF?&beta by CD4+ T�cells via the STAT5 signaling pathway
Authors: R Wang, K Huang
Mol Med Rep, 2020-04-01;0(0):.
Species: Human
Sample Types: Serum
-
MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma
Authors: Z Mei, K Zhang, AK Lam, J Huang, F Qiu, B Qiao, Y Zhang
Cancer Med, 2019-12-04;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
4D electron microscopy of T cell activation
Authors: Y Lu, BK Yoo, AHC Ng, J Kim, S Yeom, J Tang, MM Lin, AH Zewail, JR Heath
Proc. Natl. Acad. Sci. U.S.A., 2019-10-14;116(44):22014-22019.
Species: Human
Sample Types: Cell Culture Supernates
-
Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging
Authors: DP Muñoz, SM Yannone, A Daemen, Y Sun, F Vakar-Lope, M Kawahara, AM Freund, F Rodier, JD Wu, PY Desprez, DH Raulet, PS Nelson, LJ van 't Vee, J Campisi, JP Coppé
JCI Insight, 2019-06-11;5(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Sulfisoxazole inhibits the secretion of small extracellular vesicles by targeting the endothelin receptor A
Authors: EJ Im, CH Lee, PG Moon, GG Rangaswamy, B Lee, JM Lee, JC Lee, JG Jee, JS Bae, TK Kwon, KW Kang, MS Jeong, JE Lee, HS Jung, HJ Ro, S Jun, W Kang, SY Seo, YE Cho, BJ Song, MC Baek
Nat Commun, 2019-03-27;10(1):1387.
Species: Human
Sample Types: Cell Culture Supernates
-
Locally anchoring enzymes to tissues via extracellular glycan recognition
Authors: SA Farhadi, E Bracho-San, MM Fettis, DT Seroski, SL Freeman, A Restuccia, BG Keselowsky, GA Hudalla
Nat Commun, 2018-11-22;9(1):4943.
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of pepsin and pepstatin on reflux tonsil hypertrophy in vitro
Authors: JH Kim, SJ Jang, JW Yun, MH Jung, SH Woo
PLoS ONE, 2018-11-08;13(11):e0207090.
Species: Human
Sample Types: Whole Cells
-
VSIG-3 as a ligand of VISTA inhibits human T-cell function.
Authors: Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, Guan J, Singh R, Rollins S, Solorz A, Bi M, Li J, Grabowski D, Dirkx J, Tracy C, Stuart T, Ellinghuysen C, Desmond D, Foster C, Kalabokis V
Immunology, 2018-10-10;156(1):74-85.
Species: Human
Sample Types: Cell Culture Supernates
-
Altered levels of memory T cell subsets and common ?c cytokines in Strongyloides stercoralis infection and partial reversal following anthelmintic treatment
Authors: A Rajamanick, S Munisankar, Y Bhootra, CK Dolla, K Thiruvenga, TB Nutman, S Babu
PLoS Negl Trop Dis, 2018-05-24;12(5):e0006481.
Species: Human
Sample Types: Plasma
-
Anti-inflammatory cytokine and angiogenic factors levels in vitreous samples of diabetic retinopathy patients
Authors: T Tsai, S Kuehn, N Tsiampalis, MK Vu, V Kakkassery, G Stute, HB Dick, SC Joachim
PLoS ONE, 2018-03-27;13(3):e0194603.
Species: Human
Sample Types: Vitreous Humor
-
Occupational exposure to ultrafine particles in police officers: no evidence for adverse respiratory effects
Authors: G Jordakieva, I Grabovac, E Valic, KE Schmidt, A Graff, A Schuster, K Hoffmann-S, C Oberhuber, O Scheiner, A Goll, J Godnic-Cva
J Occup Med Toxicol, 2018-02-03;13(0):5.
Species: Human
Sample Types: Serum
Applications: ELISA Capture -
Construction, Expression, and Characterization of rSEA-EGF and In Vitro Evaluation of its Antitumor Activity Against Nasopharyngeal Cancer
Authors: X Liu, L Zeng, Z Zhao, Y Xie, S Wang, J Zhang, Y He, Z Zou, J Zhang, A Tao
Technol. Cancer Res. Treat., 2018-01-01;17(0):1533033818762.
Species: Human
Sample Types: Cell Culture Supernates
-
The Effects of Perioperative Anesthesia and Analgesia on Immune Function in Patients Undergoing Breast Cancer Resection: A Prospective Randomized Study
Authors: JS Cho, MH Lee, SI Kim, S Park, HS Park, E Oh, JH Lee, BN Koo
Int J Med Sci, 2017-08-18;14(10):970-976.
Species: Human
Sample Types: Serum
-
Infiltrating mast cells enhance benign prostatic hyperplasia through IL-6/STAT3/Cyclin D1 signals
Authors: Z Ou, Y He, L Qi, X Zu, L Wu, Z Cao, Y Li, L Liu, DA Dube, Z Wang, L Wang
Oncotarget, 2017-07-22;8(35):59156-59164.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocyte chemoattractant protein-1 polymorphism interaction with spirulina immunomodulatory effects in healthy Korean elderly: A 16 week, double-blind randomized clinical trial
Authors: HJ Park, HS Lee
Nutr Res Pract, 2017-06-09;11(4):290-299.
Species: Human
Sample Types: Plasma
-
Abnormal gene and protein expression of inflammatory cytokines in the postmortem brain of schizophrenia patients
Authors: GN Pandey, HS Rizavi, H Zhang, X Ren
Schizophr. Res., 2017-05-02;0(0):.
Species: Human
Sample Types: Tissue Homogenates
-
Diminished plasma levels of common ?-chain cytokines in pulmonary tuberculosis and reversal following treatment
Authors: NP Kumar, VV Banurekha, D Nair, S Babu
PLoS ONE, 2017-04-27;12(4):e0176495.
Species: Human
Sample Types: Plasma
-
T Cell Subset and Stimulation Strength-Dependent Modulation of T Cell Activation by Kv1.3 Blockers
Authors: WP Fung-Leung, W Edwards, Y Liu, K Ngo, J Angsana, G Castro, N Wu, X Liu, RV Swanson, AD Wickenden
PLoS ONE, 2017-01-20;12(1):e0170102.
Species: Human
Sample Types: Cell Culture Supernates
-
Mechanism of action and effect of immune-modulating agents in the treatment of psoriasis
Authors: Rehab M El-Gharaba
Biomed. Pharmacother, 2016-12-05;85(0):141-147.
Species: Human
Sample Types: Serum
-
Human amniotic epithelial cells inhibit CD4+ T cell activation in acute kidney injury patients by influencing the miR-101-c-Rel-IL-2 pathway
Authors: Junfeng Liu
Mol. Immunol, 2016-11-26;81(0):76-84.
Species: Human
Sample Types: Cell Lysates
-
The Suppressed Induction of Human Mature Cytotoxic T Lymphocytes Caused by Asbestos Is Not due to Interleukin-2 Insufficiency
J Immunol Res, 2016-11-15;2016(0):7484872.
Species: Human
Sample Types: Cell Culture Supernates
-
Involvement of NK Cells and NKp30 Pathway in Antisynthetase Syndrome
J Immunol, 2016-08-10;197(5):1621-30.
Species: Human
Sample Types: Serum
-
Impact of Exogenous Galectin-9 on Human T Cells: CONTRIBUTION OF THE T CELL RECEPTOR COMPLEX TO ANTIGEN-INDEPENDENT ACTIVATION BUT NOT TO APOPTOSIS INDUCTION.
Authors: Lhuillier C, Barjon C, Niki T, Gelin A, Praz F, Morales O, Souquere S, Hirashima M, Wei M, Dellis O, Busson P
J Biol Chem, 2015-05-06;290(27):16797-811.
Species: Human
Sample Types: Cell Culture Supernates
-
The Association between Serum Cytokines and Damage to Large and Small Nerve Fibers in Diabetic Peripheral Neuropathy.
Authors: Magrinelli F, Briani C, Romano M, Ruggero S, Toffanin E, Triolo G, Peter G, Praitano M, Lauriola M, Zanette G, Tamburin S
J Diabetes Res, 2015-04-16;2015(0):547834.
Species: Human
Sample Types: Serum
-
Inhibition of G-protein betagamma signaling enhances T cell receptor-stimulated interleukin 2 transcription in CD4+ T helper cells.
Authors: Yost E, Hynes T, Hartle C, Ott B, Berlot C
PLoS ONE, 2015-01-28;10(1):e0116575.
Species: Human
Sample Types: Cell Culture Supernates
-
The injury-induced myokine insulin-like 6 is protective in experimental autoimmune myositis.
Authors: Zeng L, Maruyama S, Nakamura K, Parker-Duffen J, Adham I, Zhong X, Lee H, Querfurth H, Walsh K
Skelet Muscle, 2014-08-04;4(0):16.
Species: Human
Sample Types: Cell Culture Supernates
-
Role of programmed death ligands in effective T-cell interactions in extranodal natural killer/T-cell lymphoma.
Authors: Han L, Liu F, Li R, Li Z, Chen X, Zhou Z, Zhang X, Hu T, Zhang Y, Young K, Sun S, Wen J, Zhang M
Oncol Lett, 2014-07-17;8(4):1461-1469.
Species: Human
Sample Types: Serum
-
Targeting the ion channel Kv1.3 with scorpion venom peptides engineered for potency, selectivity, and half-life.
Authors: Edwards W, Fung-Leung W, Huang C, Chi E, Wu N, Liu Y, Maher M, Bonesteel R, Connor J, Fellows R, Garcia E, Lee J, Lu L, Ngo K, Scott B, Zhou H, Swanson R, Wickenden A
J Biol Chem, 2014-06-17;289(33):22704-14.
Species: Human
Sample Types: Cell Culture Supernates
-
Activation of silenced cytokine gene promoters by the synergistic effect of TBP-TALE and VP64-TALE activators.
Authors: Anthony, Kim, More, Abhijit, Zhang, Xiaoliu
PLoS ONE, 2014-04-22;9(4):e95790.
Species: Human
Sample Types: Cell Culture Supernates
-
Sos1 regulates sustained TCR-mediated Erk activation.
Authors: Poltorak M, Meinert I, Stone J, Schraven B, Simeoni L
Eur J Immunol, 2014-02-20;44(5):1535-40.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunogenicity of a vaccine regimen composed of simian immunodeficiency virus DNA, rMVA, and viral particles administered to female rhesus macaques via four different mucosal routes.
Authors: Manrique M, Kozlowski P, Cobo-Molinos A, Wang S, Wilson R, Montefiori D, Carville A, Aldovini A
J Virol, 2013-02-13;87(8):4738-50.
Species: Human
Sample Types: Cell Culture Supernates
-
Multiplexing immunoassays for cytokine detection in the serum of patients with rheumatoid arthritis: lack of sensitivity and interference by rheumatoid factor.
Authors: Churchman SM, Geiler J, Parmar R, Horner EA, Church LD, Emery P, Buch MH, McDermott MF, Ponchel F
Clin. Exp. Rheumatol., 2012-08-29;30(4):534-42.
Species: Human
Sample Types: Serum
-
Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system.
Authors: Sun J, Fu F, Gu W, Yan R, Zhang G, Shen Z, Zhou Y, Wang H, Shen B, Zhang X
PLoS ONE, 2011-09-13;6(9):e24751.
Species: Human
Sample Types: Cell Culture Supernates
-
Antigen presentation and MHC class II expression by human esophageal epithelial cells: role in eosinophilic esophagitis.
Authors: Mulder DJ, Pooni A, Mak N, Hurlbut DJ, Basta S, Justinich CJ
Am. J. Pathol., 2011-02-01;178(2):744-53.
Species: Human
Sample Types: Cell Culture Supernates
-
HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells.
Authors: Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, Exley MA, Anderson KC, Munshi NC
Leukemia, 2010-11-19;25(1):161-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Urinary proinflammatory cytokine response in renal transplant recipients with polyomavirus BK viruria.
Authors: Sadeghi M, Daniel V, Schnitzler P, Lahdou I, Naujokat C, Zeier M, Opelz G
Transplantation, 2009-11-15;88(9):1109-16.
Species: Human
Sample Types: Urine
-
Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand.
Authors: Tieu HV, Ananworanich J, Avihingsanon A, Apateerapong W, Sirivichayakul S, Siangphoe U, Klongugkara S, Boonchokchai B, Hammer SM, Manosuthi W
AIDS Res. Hum. Retroviruses, 2009-11-01;25(11):1083-9.
Species: Human
Sample Types: Serum
-
CD200 is induced by ERK and is a potential therapeutic target in melanoma.
Authors: Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE
J. Clin. Invest., 2007-12-01;117(12):3922-3929.
Species: Human
Sample Types: Cell Culture Supernates
-
1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis.
Authors: Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR
Cytokine, 2007-10-29;40(2):128-34.
Species: Human
Sample Types: Cell Culture Supernates
-
Short communication: decreasing soluble CD30 and increasing IFN-gamma plasma levels are indicators of effective highly active antiretroviral therapy.
Authors: Sadeghi M, Susal C, Daniel V, Naujokat C, Zimmermann R, Huth-Kuhne A, Opelz G
AIDS Res. Hum. Retroviruses, 2007-07-01;23(7):886-90.
Species: Human
Sample Types: Plasma
-
Co-occurrence of IgA antibodies against ethanol metabolites and tissue transglutaminase in alcohol consumers: correlation with proinflammatory cytokines and markers of fibrogenesis.
Authors: Koivisto H, Hietala J, Anttila P, Niemela O
Dig. Dis. Sci., 2007-06-28;53(2):500-5.
Species: Human
Sample Types: Serum
-
Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer.
Authors: Zhang B, Sun T, Xue L, Han X, Zhang B, Lu N, Shi Y, Tan W, Zhou Y, Zhao D, Zhang X, Guo Y, Lin D
Carcinogenesis, 2006-12-20;28(5):1067-73.
Species: Human
Sample Types: Cell Culture Supernates
-
Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction.
Authors: Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S
Gut, 2006-07-13;56(2):243-52.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-2 in human milk: a potential modulator of lymphocyte development in the breastfed infant.
Authors: Bryan DL, Forsyth KD, Gibson RA, Hawkes JS
Cytokine, 2006-04-11;33(5):289-93.
Species: Human
Sample Types: Milk
-
Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection.
Authors: Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE
J. Immunol., 2006-03-01;176(5):3000-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Influence of eotaxin 67G>A polymorphism on plasma eotaxin concentrations in myocardial infarction survivors and healthy controls.
Authors: Sheikine Y, Olsen B, Gharizadeh B, Jatta K, Tornvall P, Ghaderi M
Atherosclerosis, 2006-02-28;189(2):458-63.
Species: Human
Sample Types: Serum
-
C-reactive protein and vascular cell adhesion molecule-1 as markers of severity in sickle cell disease.
Authors: Makis AC, Hatzimichael EC, Stebbing J, Bourantas KL
Arch. Intern. Med., 2006-02-13;166(3):366-8.
Species: Human
Sample Types: Plasma
-
Decreased plasma CXCL16/SR-PSOX concentration is associated with coronary artery disease.
Authors: Sheikine Y, Bang CS, Nilsson L, Samnegard A, Hamsten A, Jonasson L, Eriksson P, Sirsjo A
Atherosclerosis, 2005-12-27;188(2):462-6.
Species: Human
Sample Types: Serum
-
Primary cutaneous T-cell lymphomas show a deletion or translocation affecting NAV3, the human UNC-53 homologue.
Authors: Karenko L, Hahtola S, Paivinen S, Karhu R, Syrja S, Kahkonen M, Nedoszytko B, Kytölä S, Zhou Y, Blazevic V, Pesonen M, Nevala H, Nupponen N, Sihto H, Krebs I, Poustka A, Roszkiewicz J, Saksela K, Peterson P, Visakorpi T, Ranki A
Cancer Res., 2005-09-15;65(18):8101-10.
Species: Human
Sample Types: Cell Culture Supernates
-
Th2 predominance and CD8+ memory T cell depletion in patients with severe acute respiratory syndrome.
Authors: Huang JL, Huang J, Duan ZH, Wei J, Min J, Luo XH, Li JG, Tan WP, Wu LZ, Liu RY, Li Y, Shao J, Huang BJ, Zeng YX, Huang W
Microbes Infect., 2005-02-24;7(3):427-36.
Species: Human
Sample Types: Serum
-
Strong inflammatory cytokine response in male and strong anti-inflammatory response in female kidney transplant recipients with urinary tract infection.
Authors: Sadeghi M, Daniel V, Naujokat C, Wiesel M, Hergesell O, Opelz G
Transpl. Int., 2005-02-01;18(2):177-85.
Species: Human
Sample Types: Plasma
-
Cytokines and adhesion molecules in multiple sclerosis patients treated with interferon-beta1b.
Authors: Jensen J, Krakauer M, Sellebjerg F
Cytokine, 2005-01-07;29(1):24-30.
Species: Human
Sample Types: Plasma
-
Inflammatory cytokine concentrations are elevated in seminal plasma of men with spinal cord injuries.
Authors: Basu S, Aballa TC, Ferrell SM, Lynne CM, Brackett NL
J. Androl., 2004-03-01;25(2):250-4.
Species: Human
Sample Types: Seminal Plasma
-
Urinary IL-2 assay for monitoring intravesical bacillus Calmette-Guerin response of superficial bladder cancer during induction course and maintenance therapy.
Authors: Saint F, Kurth N, Maille P, Vordos D, Hoznek A, Soyeux P, Patard JJ, Abbou CC, Chopin DK
Int. J. Cancer, 2003-11-10;107(3):434-40.
Species: Human
Sample Types: Urine
-
Intraperitoneal bispecific antibody (HEA125xOKT3) therapy inhibits malignant ascites production in advanced ovarian carcinoma.
Authors: Marme A, Strauss G, Bastert G, Grischke EM, Moldenhauer G
Int. J. Cancer, 2002-09-10;101(2):183-9.
Species: Human
Sample Types: Ascites Fluid
-
Fibroblasts from human spleen regulate NK cell differentiation from blood CD34(+) progenitors via cell surface IL-15.
Authors: Briard D, Brouty-Boye D, Azzarone B, Jasmin C
J. Immunol., 2002-05-01;168(9):4326-32.
Species: Human
Sample Types: Cell Culture Supernates
-
Embryotoxicity of peritoneal fluid in women with endometriosis. Its relation with cytokines and lymphocyte populations.
Authors: Gomez-Torres MJ, Acien P, Campos A, Velasco I
Hum. Reprod., 2002-03-01;17(3):777-81.
Species: Human
Sample Types: Peritoneal Fluid
-
Local immunostimulation induced by intravesical administration of autologous interferon-gamma-activated macrophages in patients with superficial bladder cancer.
Authors: Pages F, Lebel-Binay S, Vieillefond A, Deneux L, Cambillau M, Soubrane O, Debre B, Tardy D, Lemonne JL, Abastado JP, Fridman WH, Thiounn N
Clin. Exp. Immunol., 2002-02-01;127(2):303-9.
Species: Human
Sample Types: Urine
-
Human T-cell leukemia virus type 2 induces survival and proliferation of CD34(+) TF-1 cells through activation of STAT1 and STAT5 by secretion of interferon-gamma and granulocyte macrophage-colony-stimulating factor.
Authors: Bovolenta C, Pilotti E, Mauri M, Turci M, Ciancianaini P, Fisicaro P, Bertazzoni U, Poli G, Casoli C
Blood, 2002-01-01;99(1):224-31.
Species: Human
Sample Types: Cell Culture Supernates
-
An analysis of acute changes in interleukin-6 levels after treatment of hepatitis C with consensus interferon.
Authors: Cotler SJ, Reddy KR, McCone J, Wolfe DL, Liu A, Craft TR, Ferris MW, Conrad AJ, Albrecht J, Morrissey M, Ganger DR, Rosenblate H, Blatt LM, Jensen DM, Taylor MW
J. Interferon Cytokine Res., 2001-12-01;21(12):1011-9.
Species: Human
Sample Types: Serum
-
Natural interferon alpha/beta-producing cells link innate and adaptive immunity.
Authors: Kadowaki N, Antonenko S, Lau JY, Liu YJ
J. Exp. Med., 2000-07-17;192(2):219-26.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine profiles in parotid saliva from HIV-1-infected individuals: changes associated with opportunistic infections in the oral cavity.
Authors: Black, K P, Merrill, K W, Jackson, S, Katz, J
Oral Microbiol Immunol, 2000-04-01;15(2):74-81.
Species: Human
Sample Types: Saliva
-
Effects of UVB radiation on cytokine generation, cell adhesion molecules, and cell activation markers in T-lymphocytes and peripheral blood HPCs.
Authors: Lankford KV, Mosunjac M, Hillyer CD
Transfusion, 2000-03-01;40(3):361-7.
Species: Human
Sample Types: Cell Culture Supernates
-
Human amniotic fluid lacks interleukin-2 and interleukin-15 but can interact with the beta-chain of the interleukin-2 receptor.
Authors: Searle RF, Bromage SJ, Palmer J, Curry JE, Lang AK
Immunology, 2000-03-01;99(3):411-7.
Species: Human
Sample Types: Amniotic Fluid
-
High versus low basal cortisol secretion in asymptomatic, medication-free HIV-infected men: differential effects of severe life stress on parameters of immune status.
Authors: Petitto JM, Leserman J, Perkins DO, Stern RA, Silva SG, Gettes D, Zheng B, Folds JD, Golden RN, Evans DL
Behav Med, 2000-01-01;25(4):143-51.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-2 Quantikine ELISA Kit
Average Rating: 4.7 (Based on 25 Reviews)
Have you used Human IL-2 Quantikine ELISA Kit?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
This ELISA performed as expected with low % CV's
Great assay that is easy to use and provides correct results. The typical range for healthy patients is very low, this test shows that accurately.
Used mice treated with human IL-2 product to test the IL-2 clearance in the blood.
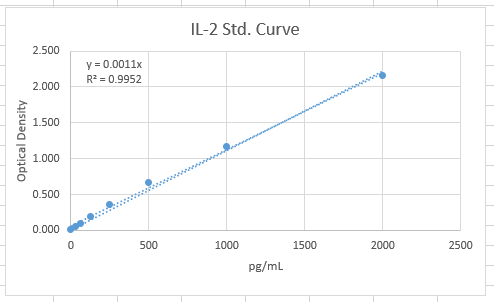
Were able to use our own IL-2 construct to make a trustable standard curve and comparable with the kit's products.
We use this kit daily.