Human IL-17 DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human IL-17. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
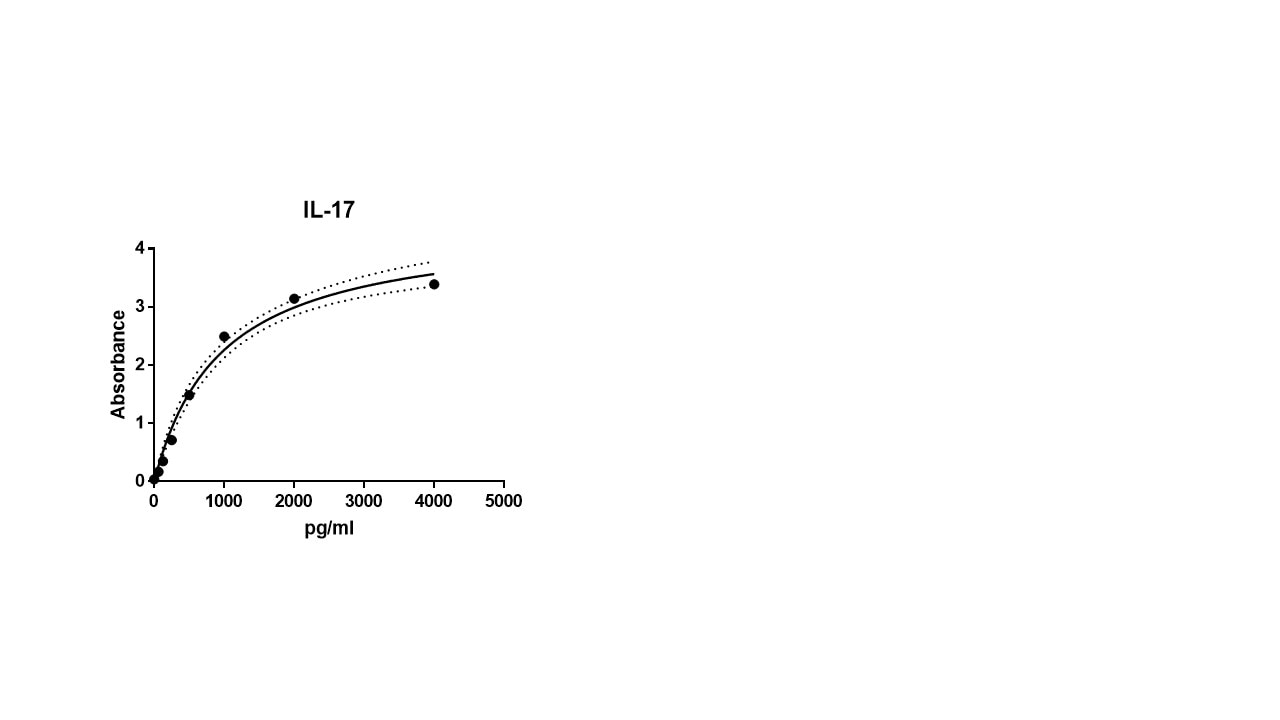
Scientific Data
Product Datasheets
Preparation and Storage
Background: IL-17/IL-17A
Human Interleukin 17 (IL-17), also known as IL-17A and CTLA-8, is a 15-20 kDa, variably glycosylated polypeptide that belongs to the IL-17 family of cytokines (1-5). Its alternate name, CTLA-8, originated from rodent studies where an activated hybridoma was created from the fusion of a mouse cytotoxic and a rat T cell lymphoma cell line. The molecule of interest in this study was assumed to have come from the mouse cytotoxic lymphocyte cell (thus the CTL designation), whereas, in fact, it was a rat lymphocyte molecule. Human IL-17/17A is synthesized as a 155 amino acid (aa) precursor that contains a 23 aa signal sequence and a 133 aa mature region that possesses a cysteine-knot fold (4-6). In both human and mouse, there is one conserved N-linked glycosylation site that likely contributes 5 kDa to its native molecular weight. IL-17A forms both a 32-38 kDa disulfide-linked homodimer, and a 40-45 kDa covalent heterodimer with IL-17F (7-9). Most secreted IL-17A is in the form of the IL-17A:F heterodimer, however, the IL-17A:A homodimer is the most bioactive of the two forms (8). Mature human IL-17A is 61%, 74%, and 99% aa identical to mouse, porcine, and chimpanzee IL-17A, respectively (10-12). Mammalian cells known to produce IL-17 are the CD4+ Th17 T cells, Paneth cells, GR1+CD11b+ myeloid suppressor cells, CD27- gamma δ T cells, CD1+NK1.1- iNKT cells, and CD3- CD4+ LTi-like cells (9, 13-17).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human IL-17 DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
74
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Interleukin 41 As A Potential Predictor of Bio-Therapy Efficacy In Patients With Rheumatoid Arthritis: A Prospective Observational Study
Authors: Joci?, J;Lu?i?, AT;Jovanovi?, I;Jurievi?, M;Stanisavljevi?, I;Stamenkovi?, B;Pavlovi?, S;
International journal of medical sciences
Species: Human
Sample Types: Serum
-
Reduced Tolerogenic Program Death-Ligand 1-Expressing Conventional Type 1 Dendritic Cells Are Associated with Rapid Decline in Chronic Obstructive Pulmonary Disease
Authors: Chen, KY;Sun, WL;Wu, SM;Feng, PH;Lin, CF;Chen, TT;Lu, YH;Ho, SC;Chen, YH;Lee, KY;
Cells
Species: Human
Sample Types: Cell Culture Supernates
-
Effects of Combinatory In Vitro Treatment with Immune Checkpoint Inhibitors and Cytarabine on the Anti-Cancer Immune Microenvironment in De Novo AML Patients
Authors: Bo?kun, ?;Starosz, A;Kr?towska-Grunwald, A;Wasiluk, T;Walewska, A;Wierzbowska, A;Moniuszko, M;Grubczak, K;
Cancers
Species: Human
Sample Types: Cell Culture Supernates
-
Significance of Th17 and Treg in Treatment Efficacy and Outcome in Pediatric Acute Lymphoblastic Leukemia
Authors: Kr?towska-Grunwald, A;Sawicka-?ukowska, M;Kowalska, M;Basaj, A;Krawczuk-Rybak, M;Moniuszko, M;Grubczak, K;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Mild Cognitive Impairment Is Associated with Enhanced Activation of Th17 Lymphocytes in Non-Alcoholic Fatty Liver Disease
Authors: Fiorillo, A;Gallego, JJ;Casanova-Ferrer, F;Giménez-Garzó, C;Urios, A;Ballester, MP;Durbán, L;Rios, MP;Megías, J;San Miguel, T;Kosenko, E;Escudero-García, D;Benlloch, S;Felipo, V;Montoliu, C;
International journal of molecular sciences
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of exogenous IL-37 on immune cells from a patient carrying a potential IL37 loss-of-function variant: A case study
Authors: LU Teufel, CI van der Ma, V Klück, A Simons, A Hoischen, V Vernimmen, LAB Joosten, RJW Arts
Cytokine, 2022-12-05;162(0):156102.
Species: Human
Sample Types: Cell Culture Supernates
-
EC-18 prevents autoimmune arthritis by suppressing inflammatory cytokines and osteoclastogenesis
Authors: JS Park, SC Yang, HY Jeong, SY Lee, JG Ryu, JW Choi, HY Kang, SM Kim, SH Hwang, ML Cho, SH Park
Arthritis Research & Therapy, 2022-11-17;24(1):254.
Species: Human
Sample Types: Serum
-
T Lymphocyte Serotonin 5-HT7 Receptor Is Dysregulated in Natalizumab-Treated Multiple Sclerosis Patients
Authors: F Reverchon, C Guillard, L Mollet, P Auzou, D Gosset, F Madouri, A Valéry, A Menuet, C Ozsancak, M Pallix-Guy, S Morisset-L
Biomedicines, 2022-09-27;10(10):.
Species: Human
Sample Types: Plasma
-
Interleukin-17, a salivary biomarker for COVID-19 severity
Authors: FS Sharif-Ask, NS Sharif-Ask, S Hafezi, B Mdkhana, HAH Alsayed, AW Ansari, B Mahboub, AM Zakeri, MH Temsah, W Zahir, Q Hamid, R Halwani
PLoS ONE, 2022-09-22;17(9):e0274841.
Species: Human
Sample Types: Plasma
-
Exercise training-induced changes in immunometabolic markers in youth badminton athletes
Authors: FE Rossi, AJ Maldonado, JM Cholewa, SLG Ribeiro, CA de Araújo, C Figueiredo, T Reichel, K Krüger, FS Lira, LG Minuzzi
Scientific Reports, 2022-09-15;12(1):15539.
Species: Human
Sample Types: Plasma
-
IL-17A in Human Liver: Significant Source of Inflammation and Trigger of Liver Fibrosis Initiation.
Authors: Kartasheva-Ebertz D, Gaston J, Lair-Mehiri L, Mottez E, Buivan T, Massault P, Scatton O, Gaujoux S, Vaillant J, Pol S, Lagaye S
Int J Mol Sci, 2022-08-29;23(17):.
Species: Human
Sample Types: Tissue Culture Supernates
-
Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via TGR5 signaling
Authors: J Hu, Y Zhang, S Yi, C Wang, X Huang, S Pan, J Yang, G Yuan, S Tan, H Li
International journal of biological sciences, 2022-07-11;18(11):4545-4559.
Species: Human
Sample Types: Cell Culture Supernates
-
High-intensity intermittent exercise induces a potential anti-inflammatory response in healthy women across the menstrual cycle
Authors: LG Minuzzi, FS Lira, RAB de Poli, VH Fialho Lop, AM Zagatto, K Suzuki, BM Antunes
Cytokine, 2022-04-08;154(0):155872.
Species: Human
Sample Types: Serum
-
A novel Streptococcus pneumoniae human challenge model demonstrates Treg lymphocyte recruitment to the infection site
Authors: G Szylar, R Wysoczansk, H Marshall, DJB Marks, R José, ME Ehrenstein, JS Brown
Scientific Reports, 2022-03-07;12(1):3990.
Species: Human
Sample Types: Blister Fluid
-
Vitamin D3 and zinc synergistically induce regulatory T cells and suppress interferon-gamma production in mixed lymphocyte culture
Authors: AK Schmitt, MA Puppa, I Wessels, L Rink
The Journal of nutritional biochemistry, 2022-01-19;0(0):108942.
Species: Human
Sample Types: Cell Culture Supernates
-
Skin Substitute Preparation Method Induces Immunomodulatory Changes in Co-Incubated Cells through Collagen Modification
Authors: J Holl, C Pawlukiani, J Corton Rui, D Groth, K Grubczak, HR Hady, J Dadan, J Reszec, S Czaban, C Kowalewski, M Moniuszko, A Eljaszewic
Pharmaceutics, 2021-12-15;13(12):.
Species: Human
Sample Types: Cell Culture Supernates
-
Bacterial genotoxins induce T�cell senescence
Authors: SL Mathiasen, L Gall-Mas, IS Pateras, SDP Theodorou, MRJ Namini, MB Hansen, OCB Martin, CK Vadivel, K Ntostoglou, D Butter, M Givskov, C Geisler, AN Akbar, VG Gorgoulis, T Frisan, N Ødum, T Krejsgaard
Cell Reports, 2021-06-08;35(10):109220.
Species: Human
Sample Types: Cell Culture Supernates
-
NLRP1 acts as a negative regulator of Th17 cell programming in mice and humans with autoimmune diabetes
Authors: FRC Costa, JA Leite, DM Rassi, JF da Silva, J Elias-Oliv, JB Guimarães, MC Foss-Freit, NOS Câmara, A Pontillo, RC Tostes, JS Silva, D Carlos
Cell Reports, 2021-05-25;35(8):109176.
Species: Human
Sample Types: Serum
-
Higher CD4/CD8 ratio of pleural effusion predicts better survival for lung cancer patients receiving immune checkpoint inhibitors
Authors: PH Lee, TY Yang, KC Chen, YH Huang, JS Tseng, KH Hsu, YC Wu, KJ Liu, GC Chang
Scientific Reports, 2021-04-30;11(1):9381.
Species: Human
Sample Types: Pleural Effusion
-
Electrophilic nitro-fatty acids suppress psoriasiform dermatitis: STAT3 inhibition as a contributory mechanism
Authors: P Wang, ME Killeen, TL Sumpter, LK Ferris, LD Falo, BA Freeman, FJ Schopfer, AR Mathers
Redox Biology, 2021-04-24;43(0):101987.
Species: Human
Sample Types: Cell Culture Supernates
-
New Biomarker in Chagas Disease: Extracellular Vesicles Isolated from Peripheral Blood in Chronic Chagas Disease Patients Modulate the Human Immune Response
Authors: RP Madeira, LM Dal'Mas Ro, P de Cássia, C Mady, BM Ianni, AC Torrecilha
Journal of Immunology Research, 2021-01-11;2021(0):6650670.
Species: Human
Sample Types: Cell Culture Supernates
-
SARS-CoV-2-specific IgA and limited inflammatory cytokines are present in the stool of select patients with acute COVID-19
Authors: GJ Britton, A Chen-Liaw, F Cossarini, AE Livanos, MP Spindler, T Plitt, J Eggers, I Mogno, A Gonzalez-R, S Siu, M Tankelevic, L Grinspan, RE Dixon, D Jha, G Martinez-D, F Amanat, DA Hoagland, B tenOever, MC Dubinsky, M Merad, H van Bakel, F Krammer, G Bongers, S Mehandru, JJ Faith
medRxiv : the preprint server for health sciences, 2020-12-09;0(0):.
Species: Human
Sample Types: Fecal Supernates
-
The DHODH Inhibitor PTC299 Arrests SARS-CoV-2 Replication and Suppresses Induction of Inflammatory Cytokines
Authors: J Luban, R Sattler, E Mühlberger, JD Graci, L Cao, M Weetall, C Trotta, JM Colacino, S Bavari, C Strambio-D, EL Suder, Y Wang, V Soloveva, K Cintron-Lu, NA Naryshkin, M Pykett, EM Welch, K O'Keefe, R Kong, E Goodwin, A Jacobson, S Paessler, S Peltz
Virus Res, 2020-11-26;0(0):198246.
Species: Human
Sample Types: Cell Culture Supernates
-
Monocytes differentiated into macrophages and dendritic cells in the presence of human IFN-&lambda3 or IFN-&lambda4 show distinct phenotypes
Authors: M De, A Bhushan, S Chinnaswam
J Leukoc Biol, 2020-11-17;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of EMMPRIN/CD147 in regulating angiogenesis in patients with psoriatic arthritis
Authors: MA Rahat, M Safieh, E Simanovich, E Pasand, T Gazitt, A Haddad, M Elias, D Zisman
Arthritis Res Ther, 2020-10-14;22(1):240.
Species: Human
Sample Types: Serum
-
Lung immunoglobulin A immunity dysregulation in cystic fibrosis
Authors: AM Collin, M Lecocq, S Noel, B Detry, FM Carlier, F Aboubakar, C Bouzin, T Leal, M Vermeersch, V De Rose, L Regard, C Martin, PR Burgel, D Hoton, S Verleden, A Froidure, C Pilette, S Gohy
EBioMedicine, 2020-09-11;60(0):102974.
Species: Human
Sample Types: Sputum Supernates
-
Erythromycin inhibits neutrophilic inflammation and mucosal disease by upregulating DEL-1
Authors: T Maekawa, H Tamura, H Domon, T Hiyoshi, T Isono, D Yonezawa, N Hayashi, N Takahashi, K Tabeta, T Maeda, M Oda, A Ziogas, V? Alexaki, T Chavakis, Y Terao, G Hajishenga
JCI Insight, 2020-08-06;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
FTY720 ameliorates GvHD by blocking T lymphocyte migration to target organs and by skin fibrosis inhibition
Authors: J Ryu, J Jhun, MJ Park, JA Baek, SY Kim, KH Cho, JW Choi, SH Park, JY Choi, ML Cho
J Transl Med, 2020-06-06;18(1):225.
Species: Human
Sample Types: Cell Culture Supernates
-
Gaps in Study Design for Immune Parameter Research for Latent Tuberculosis Infection: A Systematic Review
Authors: M Herrera, C Vera, Y Keynan, ZV Rueda
J Immunol Res, 2020-04-21;2020(0):8074183.
Species: Human
Sample Types: Cell Culture Supernates
-
1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis
Authors: H Kim, S Baek, SM Hong, J Lee, SM Jung, J Lee, M Cho, SK Kwok, SH Park
J. Korean Med. Sci., 2020-02-17;35(6):e40.
Species: Human
Sample Types: Cell Culture Supernates
-
Locally-secreted interleukin-6 is related with radiological severity in smear-negative pulmonary tuberculosis
Authors: PX Losada, F Perdomo-Ce, M Castro, C Salcedo, A Salcedo, I DeLaura, G Lastra, CF Narváez
Cytokine, 2019-12-25;127(0):154950.
Species: Human
Sample Types: BALF
-
Replication of Genome-Wide Association Analysis Identifies New Susceptibility Loci at Long Noncoding RNA Regions for Vogt-Koyanagi-Harada Disease
Authors: J Qi, L Du, J Deng, Y Qin, G Su, S Hou, M Lv, Q Zhang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2019-11-01;60(14):4820-4829.
Species: Human
Sample Types: Cell Culture Supernates
-
Type 1 diabetic mellitus patients with increased atherosclerosis risk display decreased CDKN2A/2B/2BAS gene expression in leukocytes
Authors: S Martínez-H, V Sánchez-Ga, A Herrero-Ce, Á Vinué, JT Real, JF Ascaso, DJ Burks, H González-N
J Transl Med, 2019-07-12;17(1):222.
Species: Human
Sample Types: Plasma
-
PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia
Authors: T Lopatina, E Favaro, C Grange, M Cedrino, A Ranghino, S Occhipinti, S Fallo, F Buffolo, DA Gaykalova, MM Zanone, R Romagnoli, G Camussi
Sci Rep, 2018-12-04;8(1):17458.
Species: Human
Sample Types: Cell Culture Supernates
-
Disabled-2 (DAB2) Overexpression Inhibits Monocyte-Derived Dendritic Cells' Function in Vogt-Koyanagi-Harada Disease
Authors: S Yi, R Chang, J Hu, Y Qiu, Q Wang, Q Cao, G Yuan, G Su, C Zhou, Y Wang, A Kijlstra, P Yang
Invest. Ophthalmol. Vis. Sci., 2018-09-04;59(11):4662-4669.
Species: Human
Sample Types: Cell Culture Supernates
-
Association of genetic variations in PTPN2 and CD122 with ocular Behcet's disease
Authors: Q Zhang, H Li, S Hou, H Yu, G Su, B Deng, J Qi, C Zhou, A Kijlstra, P Yang
Br J Ophthalmol, 2018-03-03;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cholera toxin enhances IL-17A production in both CD4and CD8cells via a cAMP/PKA-mediated IL-17A promoter activation
Authors: HC Tsa, S Velichko, S Lee, R Wu
Immunology, 2018-02-20;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis
Authors: M Manetti, S Pratesi, E Romano, S Bellando-R, I Rosa, S Guiducci, BS Fioretto, L Ibba-Manne, E Maggi, M Matucci-Ce
PLoS ONE, 2017-08-10;12(8):e0183102.
Species: Human
Sample Types: Serum
-
IFN-?1 with Th17 axis cytokines and IFN-? define different subsets in systemic lupus erythematosus (SLE)
Authors: V Oke, S Brauner, A Larsson, J Gustafsson, A Zickert, I Gunnarsson, E Svenungsso
Arthritis Res. Ther., 2017-06-15;19(1):139.
Species: Human
Sample Types: Serum
-
TB lymphadenitis is associated with enhanced baseline and antigen - specific induction of Type 1 and Type 17 cytokines and reduced IL-1? and IL-18 at the site of infection
Authors: GR Kathamuthu, K Moideen, D Baskaran, VV Banurekha, D Nair, G Sekar, R Sridhar, B Vidyajayan, G Gajendrara, DK Parandhama, A Srinivasan, S Babu
Clin. Vaccine Immunol, 2017-05-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
The heterogeneity of systemic inflammation in bronchiectasis
Authors: AD Saleh, JD Chalmers, A De Soyza, TC Fardon, SO Koustas, J Scott, AJ Simpson, JS Brown, JR Hurst
Respir Med, 2017-04-17;127(0):33-39.
Species: Human
Sample Types: Serum
-
Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype
Authors: SJ Snelling, S Bas, GJ Puskas, SG Dakin, D Suva, A Finckh, C Gabay, P Hoffmeyer, AJ Carr, A Lübbeke
PLoS ONE, 2017-04-11;12(4):e0175109.
Species: Human
Sample Types: Serum
-
Berberine exerts an anti-inflammatory role in ocular Behcet's disease
Mol Med Rep, 2016-12-05;15(1):97-102.
Species: Human
Sample Types: Cell Culture Supernates
-
Interaction among activated lymphocytes and mesenchymal cells through podoplanin is critical for a high IL-17 secretion
Authors: Mélissa Noack
Arthritis Res Ther, 2016-06-23;18(0):148.
Species: Human
Sample Types: Cell Culture Supernates
-
Analysis of receptor tyrosine kinase genetics identifies two novel risk loci in GAS6 and PROS1 in Beh�et's disease
Sci Rep, 2016-05-25;6(0):26662.
Species: Human
Sample Types: Cell Culture Supernates
-
Stromal fibroblasts support dendritic cells to maintain IL-23/Th17 responses after exposure to ionizing radiation
Authors: A Malecka, Q Wang, S Shah, RV Sutavani, I Spendlove, JM Ramage, J Greensmith, HA Franks, MJ Gough, A Saalbach, PM Patel, AM Jackson
J Leukoc Biol, 2016-04-05;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Cooperation between IL-7 Receptor and Integrin alpha2beta1 (CD49b) Drives Th17-Mediated Bone Loss.
Authors: El Azreq M, Arseneault C, Boisvert M, Page N, Allaeys I, Poubelle P, Tessier P, Aoudjit F
J Immunol, 2015-09-25;195(9):4198-209.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-23 Receptor Gene Polymorphism May Enhance Expression of the IL-23 Receptor, IL-17, TNF-alpha and IL-6 in Behcet's Disease.
Authors: Jiang Z, Hennein L, Tao Y, Tao L
PLoS ONE, 2015-07-29;10(7):e0134632.
Species: Human
Sample Types: Cell Culture Supernates
-
Cytokine profiles at admission can be related to outcome in AIDS patients with cryptococcal meningitis.
Authors: Mora D, Fortunato L, Andrade-Silva L, Ferreira-Paim K, Rocha I, Vasconcelos R, Silva-Teixeira D, Nascentes G, Silva-Vergara M
PLoS ONE, 2015-03-23;10(3):e0120297.
Species: Human
Sample Types: Serum
-
A functional variant of PTPN22 confers risk for Vogt-Koyanagi-Harada syndrome but not for ankylosing spondylitis.
Authors: Zhang Q, Qi J, Hou S, DU L, Yu H, Cao Q, Zhou Y, Liao D, Kijlstra A, Yang P
PLoS ONE, 2014-05-09;9(5):e96943.
Species: Human
Sample Types: Cell Culture Supernates
-
The role of interleukin-1 receptor-associated kinases in Vogt-Koyanagi-Harada disease.
Authors: Sun M, Yang P, DU L, Yang Y, Ye J
PLoS ONE, 2014-04-01;9(4):e93214.
Species: Human
Sample Types: Cell Culture Supernates
-
HIF-1alpha- and hypoxia-dependent immune responses in human CD4+CD25high T cells and T helper 17 cells.
Authors: Bollinger T, Gies S, Naujoks J, Feldhoff L, Bollinger A, Solbach W, Rupp J
J Leukoc Biol, 2014-03-24;96(2):305-12.
Species: Human
Sample Types: Cell Culture Supernates
-
Immunoepidemiological profiling of onchocerciasis patients reveals associations with microfilaria loads and ivermectin intake on both individual and community levels.
Authors: Arndts, Kathrin, Specht, Sabine, Debrah, Alexande, Tamarozzi, Francesc, Klarmann Schulz, Ute, Mand, Sabine, Batsa, Linda, Kwarteng, Alexande, Taylor, Mark, Adjei, Ohene, Martin, Coralie, Layland, Laura E, Hoerauf, Achim
PLoS Negl Trop Dis, 2014-02-20;8(2):e2679.
Species: Human
Sample Types: Cell Culture Supernates
-
The aryl hydrocarbon receptor is functionally upregulated early in the course ofvhuman T-cell activation.
Authors: Prigent L, Robineau M, Jouneau S, Morzadec C, Louarn L, Vernhet L, Fardel O, Sparfel L
Eur J Immunol, 2014-02-18;44(5):1330-40.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN- alpha blocks IL-17 production by peripheral blood mononuclear cells in patients with chronic active hepatitis B Infection.
Authors: Cui F, Meng J, Luo P, Chen P
BMC Infect Dis, 2014-02-01;14(0):55.
Species: Human
Sample Types: Cell Culture Supernates
-
CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells.
Authors: Cheng K, Wong S, Linn Y, Ho L, Chng W, Schwarz H
Br J Haematol, 2014-01-15;165(1):134-44.
Species: Human
Sample Types: Cell Culture Supernates
-
TNF-alpha blockade induces IL-10 expression in human CD4+ T cells.
Authors: Evans H, Roostalu U, Walter G, Gullick N, Frederiksen K, Roberts C, Sumner J, Baeten D, Gerwien J, Cope A, Geissmann F, Kirkham B, Taams L
Nat Commun, 2014-01-01;5(0):3199.
Species: Human
Sample Types: Cell Culture Supernates
-
Independent and interdependent immunoregulatory effects of IL-27, IFN-beta, and IL-10 in the suppression of human Th17 cells and murine experimental autoimmune encephalomyelitis.
Authors: Fitzgerald D, Fonseca-Kelly Z, Cullimore M, Safabakhsh P, Saris C, Zhang G, Rostami A
J Immunol, 2013-03-01;190(7):3225-34.
Species: Human
Sample Types: Cell Culture Supernates
-
CCR2+CCR5+ T Cells Produce Matrix Metalloproteinase-9 and Osteopontin in the Pathogenesis of Multiple Sclerosis.
Authors: Sato W, Tomita A, Ichikawa D, Lin Y, Kishida H, Miyake S, Ogawa M, Okamoto T, Murata M, Kuroiwa Y, Aranami T, Yamamura T
J Immunol, 2012-10-15;189(10):5057-65.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated adaptive immune responses are associated with latent infections of Wuchereria bancrofti.
Authors: Arndts K, Deininger S, Specht S, Klarmann U, Mand S, Adjobimey T, Debrah AY, Batsa L, Kwarteng A, Epp C, Taylor M, Adjei O, Layland LE, Hoerauf A
PLoS Negl Trop Dis, 2012-04-03;6(4):e1611.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist.
Authors: van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, Van der Meer JW, Hao R, Kalabokis V, Dinarello CA
Proc. Natl. Acad. Sci. U.S.A., 2012-02-06;109(8):3001-5.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated serum osteopontin levels and genetic polymorphisms of osteopontin are associated with Vogt-Koyanagi-Harada disease.
Authors: Chu M, Yang P, Hu R, Hou S, Li F, Chen Y, Kijlstra A
Invest. Ophthalmol. Vis. Sci., 2011-09-09;52(10):7084-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum levels of Th17-related cytokines in Behcet disease patients after cataract surgery.
Authors: Jiang S, Liu X, Luo L, Qu B, Huang X, Lin Y, Ye S, Liu Y
Mol. Vis., 2011-05-31;17(0):1425-30.
Species: Human
Sample Types: Serum
-
IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells.
Authors: Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, Kelly PJ, Costelloe L, Tubridy N, Mills KH, Fletcher JM
Brain Behav. Immun., 2011-03-21;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
A naturally occurring, soluble antagonist of human IL-23 inhibits the development and in vitro function of human Th17 cells.
Authors: Yu RY, Gallagher G
J. Immunol., 2010-11-12;185(12):7302-8.
Species: Human
Sample Types: Cell Culture Supernates
-
IFN-alpha blocks IL-17 production by peripheral blood mononuclear cells in Behcet's disease.
Authors: Liu X, Yang P, Wang C, Li F, Kijlstra A
Rheumatology (Oxford), 2010-11-08;50(2):293-8.
Species: Human
Sample Types: Cell Culture Supernates
-
Naive and activated T cells display differential responsiveness to TL1A that affects Th17 generation, maintenance, and proliferation.
Authors: Jones GW, Stumhofer JS, Foster T, Twohig JP, Hertzog P, Topley N, Williams AS, Hunter CA, Jenkins BJ, Wang EC, Jones SA
FASEB J., 2010-09-08;25(1):409-19.
Species: Human
Sample Types: Cell Culture Supernates
-
TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23.
Authors: Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M
Eur. J. Immunol., 2009-05-01;39(5):1221-30.
Species: Human
Sample Types: Cell Culture Supernates
-
Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells.
Authors: Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G
Circulation, 2009-03-02;119(10):1424-32.
Species: Human
Sample Types: Plasma
-
Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin.
Authors: Johansen C, Usher PA, Kjellerup RB
Br. J. Dermatol., 2008-10-21;160(2):319-24.
Species: Human
Sample Types: Tissue Homogenates
-
Diminished frequency and function of CD4+CD25high regulatory T cells associated with active uveitis in Vogt-Koyanagi-Harada syndrome.
Authors: Chen L, Yang P, Zhou H, He H, Ren X, Chi W, Wang L, Kijlstra A
Invest. Ophthalmol. Vis. Sci., 2008-04-17;49(8):3475-82.
Species: Human
Sample Types: Cell Culture Supernates
-
Cutting edge: Human Th17 cells are identified as bearing CCR2+CCR5- phenotype.
Authors: Sato W, Aranami T, Yamamura T
J. Immunol., 2007-06-15;178(12):7525-9.
Species: Human
Sample Types: Cell Culture Supernates
-
Blockade of TLR9 agonist-induced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC.
Authors: Meyers JA, Mangini AJ, Nagai T, Roff CF, Sehy D, van Seventer JM
Cytokine, 2006-10-18;35(5):235-46.
Species: Human
Sample Types: Cell Culture Supernates
-
IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate.
Authors: Miranda-Carus ME, Balsa A, Benito-Miguel M, Perez de Ayala C, Martin-Mola E
J. Immunol., 2004-07-15;173(2):1463-76.
Species: Human
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human IL-17 DuoSet ELISA
Average Rating: 4.7 (Based on 3 Reviews)
Have you used Human IL-17 DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Easy to use protocol and great results