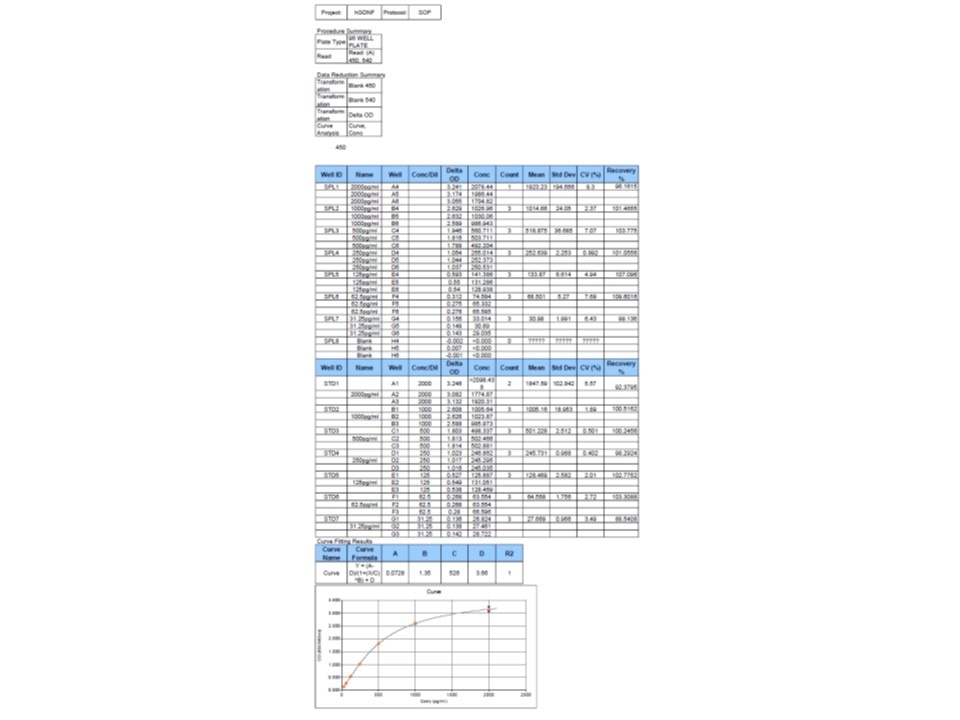
Human GDNF DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human GDNF. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
Scientific Data
Product Datasheets
Preparation and Storage
Background: GDNF
GDNF (Glial Cell Line-derived Neurotrophic Factor) is a secreted protein that promotes the survival of motoneurons, midbrain dopaminergic neurons, Purkinje cells, and sympathetic neurons. It is produced by Sertoli cells, type 1 astrocytes, Schwann cells, neurons, pinealocytes, and skeletal muscle cells. GDNF binding to GFR alpha 1 induces the recruitment of Ret, NCAM-1/CD56, various integrins, Syndecan-3, or N-Cadherin. GDNF-based therapies show promise in several neurodegenerative disorders, particularly Parkinson’s disease.
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Human GDNF DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
39
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Chronic hyperpalatable diet induces impairment of hippocampal-dependent memories and alters glutamatergic and fractalkine axis signaling
Authors: Ribeiro, R;Silva, EG;Moreira, FC;Gomes, GF;Cussat, GR;Silva, BSR;da Silva, MCM;de Barros Fernandes, H;de Sena Oliveira, C;de Oliveira Guarnieri, L;Lopes, V;Ferreira, CN;de Faria, AMC;Maioli, TU;Ribeiro, FM;de Miranda, AS;Moraes, GSP;de Oliveira, ACP;Vieira, LB;
Scientific reports
Species: Mouse
Sample Types: Serum, Tissue Homogenates
-
GDNF gene therapy for alcohol use disorder in male non-human primates
Authors: Ford, MM;George, BE;Van Laar, VS;Holleran, KM;Naidoo, J;Hadaczek, P;Vanderhooft, LE;Peck, EG;Dawes, MH;Ohno, K;Bringas, J;McBride, JL;Samaranch, L;Forsayeth, JR;Jones, SR;Grant, KA;Bankiewicz, KS;
Nature medicine
Species: Primate - Rhesus macaque
Sample Types: Tissue Homogenates
-
Composite Coatings Based on Recombinant Spidroins and Peptides with Motifs of the Extracellular Matrix Proteins Enhance Neuronal Differentiation of Neural Precursor Cells Derived from Human Induced Pluripotent Stem Cells
Authors: EV Novosadova, OV Dolotov, LV Novosadova, LI Davydova, KV Sidoruk, EL Arsenyeva, DM Shimchenko, VG Debabov, VG Bogush, VZ Tarantul
International Journal of Molecular Sciences, 2023-03-02;24(5):.
Species: Human
Sample Types: Cell Culture Supernates
-
Glial Cell Line-Derived Neurotrophic Factor-Loaded CMCht/PAMAM Dendrimer Nanoparticles for Peripheral Nerve Repair
Authors: A Escobar, MR Carvalho, FR Maia, RL Reis, TH Silva, JM Oliveira
Pharmaceutics, 2022-11-08;14(11):.
Species: Human
Sample Types: Cell Culture Supernates
-
Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial
Authors: RH Baloh, JP Johnson, P Avalos, P Allred, S Svendsen, G Gowing, K Roxas, A Wu, B Donahue, S Osborne, G Lawless, B Shelley, K Wheeler, C Prina, D Fine, T Kendra-Rom, H Stokes, V Manoukian, A Muthukumar, L Garcia, MG Bañuelos, M Godoy, C Bresee, H Yu, D Drazin, L Ross, R Naruse, H Babu, EA Macklin, A Vo, A Elsayegh, W Tourtellot, M Maya, M Burford, F Diaz, CG Patil, RA Lewis, CN Svendsen
Nature Medicine, 2022-09-05;28(9):1813-1822.
Species: Rat
Sample Types: Tissue Homogenates
-
Human Primary Astrocytes Differently Respond to Pro- and Anti-Inflammatory Stimuli
Authors: P Szpakowski, D Ksiazek-Wi, M Turniak-Ku, I Pacan, A Glabinski
Biomedicines, 2022-07-22;10(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Effect of Chemotherapy Cytarabine and Acute Myeloid Leukemia on the Development of Spermatogenesis at the Adult Age of Immature Treated Mice
Authors: B Khaleel, E Lunenfeld, J Kapelushni, M Huleihel
International Journal of Molecular Sciences, 2022-04-04;23(7):.
Species: Human
Sample Types: Tissue Homogenates
-
New Therapy for Spinal Cord Injury: Autologous Genetically-Enriched Leucoconcentrate Integrated with Epidural Electrical Stimulation
Authors: R Islamov, F Bashirov, A Izmailov, F Fadeev, V Markosyan, M Sokolov, M Shmarov, D Logunov, B Naroditsky, I Lavrov
Cells, 2022-01-02;11(1):.
Species: Human
Sample Types: Cell Culture Supernates
-
Peripheral Nerve-Derived Stem Cell Spheroids Induce Functional Recovery and Repair after Spinal Cord Injury in Rodents
Authors: HL Lee, CE Yeum, H Lee, J Oh, JT Kim, WJ Lee, Y Ha, YI Yang, KN Kim
International Journal of Molecular Sciences, 2021-04-16;22(8):.
Species: Human
Sample Types: Cell Culture Supernates
-
Circulating neurotrophins and hemostatic risk factors of atherothrombotic cardiovascular disease at baseline and during sympathetic challenge: the SABPA study
Authors: R von Känel, M Hamer, A Wentzel, L Malan
Scientific Reports, 2021-01-27;11(1):2297.
Species: Human
Sample Types: Serum
-
Unmasking a new prognostic marker and therapeutic target from the GDNF-RET/PIT1/p14ARF/p53 pathway in acromegaly
Authors: M Chenlo, IA Rodriguez-, R Serramito, AR Garcia-Ren, R Villar-Tai, E Fernandez-, S Perez-Rome, M Suarez-Far, A Garcia-All, JM Cabezas-Ag, J Rodriguez-, PV Lear, RM Alvarez-Sa, C Alvarez-Es, I Bernabeu, CV Alvarez
EBioMedicine, 2019-04-08;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Long-term, targeted delivery of GDNF from encapsulated cells is neuroprotective and reduces seizures in the pilocarpine model of epilepsy
Authors: G Paolone, C Falcicchia, F Lovisari, M Kokaia, WJ Bell, T Fradet, M Barbieri, LU Wahlberg, DF Emerich, M Simonato
J. Neurosci., 2019-01-21;0(0):.
Species: Human
Sample Types: Cell Culture Supernates
-
Serum nerve growth factor beta, brain- and glial-derived neurotrophic factor levels and psychopathology in unmedicated patients with schizophrenia
Authors: CS Chu, CL Chu, CC Wu, T Lu
J Chin Med Assoc, 2018-02-01;0(0):.
Species: Human
Sample Types: Serum
-
miRNA profiling of NurOwn�: mesenchymal stem cells secreting neurotrophic factors
Authors: Y Gothelf, H Kaspi, N Abramov, R Aricha
Stem Cell Res Ther, 2017-11-07;8(1):249.
Species: Human
Sample Types: Cell Culture Supernates
-
Elevated glutamate and lactate predict brain death after severe head trauma
Authors: MA Stefani, R Modkovski, G Hansel, ER Zimmer, A Kopczynski, AP Muller, NR Strogulski, MS Rodolphi, RK Carteri, AP Schmidt, JP Oses, DH Smith, LV Portela
Ann Clin Transl Neurol, 2017-05-04;4(6):392-402.
Species: Human
Sample Types: CSF
-
A Novel Neuroprotective Small Molecule for Glial Cell Derived Neurotrophic Factor Induction and Photoreceptor Rescue
Authors: P Baranov, H Lin, K McCabe, D Gale, S Cai, B Lieppman, D Morrow, P Lei, J Liao, M Young
J Ocul Pharmacol Ther, 2017-04-25;0(0):.
Species: Human, Mouse
Sample Types: Cell Culture Supernates, Tissue Homogenates
-
Melatonin antagonizes interleukin-18-mediated inhibition on neural stem cell proliferation and differentiation
Authors: Z Li, X Li, MTV Chan, WKK Wu, D Tan, J Shen
J. Cell. Mol. Med., 2017-04-21;0(0):.
Species: Rat
Sample Types: Cell Culture Supernates
-
Systemic injection of AAV9-GDNF provides modest functional improvements in the SOD1(G93A) ALS rat but has adverse side effects
Authors: GM Thomsen, M Alkaslasi, JP Vit, G Lawless, M Godoy, G Gowing, O Shelest, CN Svendsen
Gene Ther, 2017-03-09;0(0):.
Species: Rat
Sample Types: Tissue Homogenates
-
Serum levels of neurotrophic factors in active toxoplasmic retinochoroiditis
Authors: Cynthia Azeredo Cordeiro
Braz J Infect Dis, 2016-12-05;0(0):.
Species: Human
Sample Types: Serum
-
NG2 expression in microglial cells affects the expression of neurotrophic and proinflammatory factors by regulating FAK phosphorylation
Authors: Lie Zhu
Sci Rep, 2016-06-16;6(0):27983.
Species: Mouse, Rat
Sample Types: Cell Culture Supernates, Serum
-
Age and lesion-induced increases of GDNF transgene expression in brain following intracerebral injections of DNA nanoparticles.
Authors: Yurek D, Hasselrot U, Cass W, Sesenoglu-Laird O, Padegimas L, Cooper M
Neuroscience, 2014-10-22;284(0):500-12.
Species: Human
Sample Types: Tissue Homogenates
-
Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model.
Authors: Kim, Kwang S, Lee, Hong J, An, Jin, Kim, Yun B, Ra, Jung Cha, Lim, Inja, Kim, Seung U
Cell Transplant, 2013-09-18;23(12):1585-97.
Species: Human
Sample Types: Cell Culture Supernates
-
Repeated systemic administration of human adipose-derived stem cells attenuates overt diabetic nephropathy in rats.
Authors: Zhang L, Li K, Liu X, Li D, Luo C, Fu B, Cui S, Zhu F, Zhao R, Chen X
Stem Cells Dev, 2013-08-21;22(23):3074-86.
Species: Human
Sample Types: Cell Culture Supernates
-
A Monoclonal Antibody-GDNF Fusion Protein Is Not Neuroprotective and Is Associated with Proliferative Pancreatic Lesions in Parkinsonian Monkeys.
Authors: Ohshima-Hosoyama S, Simmons HA, Goecks N
PLoS ONE, 2012-06-20;7(6):e39036.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Tissue Homogenates
-
Non-viral gene therapy for GDNF production in RCS rat: the crucial role of the plasmid dose.
Gene Ther., 2011-10-13;19(9):886-98.
Species: Rat
Sample Types: Cell Culture Supernates
-
Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson's disease.
Authors: Glavaski-Joksimovic A, Virag T, Mangatu TA
J. Neurosci. Res., 2010-09-01;88(12):2669-81.
Species: Human
Sample Types: Cell Culture Supernates
-
Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity.
Authors: Jones JL, Anderson JM, Phuah CL, Fox EJ, Selmaj K, Margolin D, Lake SL, Palmer J, Thompson SJ, Wilkins A, Webber DJ, Compston DA, Coles AJ
Brain, 2010-07-21;133(0):2232-47.
Species: Human
Sample Types: Cell Culture Supernates
-
Lack of humoral immune response to the tetracycline (Tet) activator in rats injected intracranially with Tet-off rAAV vectors.
Authors: Han Y, Chang QA, Virag T
Gene Ther., 2010-02-18;17(5):616-25.
Species: Rat
Sample Types: Tissue Homogenates
-
Pharmacokinetics and bioactivity of glial cell line-derived factor (GDNF) and neurturin (NTN) infused into the rat brain.
Authors: Hadaczek P, Johnston L, Forsayeth J, Bankiewicz KS
Neuropharmacology, 2010-02-11;58(7):1114-21.
Species: Rat
Sample Types: Tissue Homogenates
-
Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF.
Authors: Emborg ME, Moirano J, Raschke J, Bondarenko V, Zufferey R, Peng S, Ebert AD, Joers V, Roitberg B, Holden JE, Koprich J, Lipton J, Kordower JH, Aebischer P
Neurobiol. Dis., 2009-08-04;36(2):303-11.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Tissue Homogenates
-
Effect of nigrostriatal damage induced by 6-hydroxydopamine on the expression of glial cell line-derived neurotrophic factor in the striatum of the rat.
Authors: Mertens B, Massie A, Michotte Y, Sarre S
Neuroscience, 2009-04-19;162(1):148-54.
Species: Rat
Sample Types: Tissue Homogenates
-
Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS.
Authors: Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN
Mol. Ther., 2008-09-16;16(12):2002-10.
Species: Human
Sample Types: Cell Culture Supernates
-
Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury.
Authors: Wang YC, Wu YT, Huang HY, Lin HI, Lo LW, Tzeng SF, Yang CS
Biomaterials, 2008-09-06;29(34):4546-53.
Species: Human
Sample Types: Recombinant Protein
-
Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson disease.
Authors: Sajadi A, Bensadoun JC, Schneider BL, Lo Bianco C, Aebischer P
Neurobiol. Dis., 2005-11-21;22(1):119-29.
Species: Human
Sample Types: Cell Culture Supernates
-
Neurotrophic factors in relapsing remitting and secondary progressive multiple sclerosis patients during interferon beta therapy.
Authors: Caggiula M, Batocchi AP, Frisullo G, Angelucci F, Patanella AK, Sancricca C, Nociti V, Tonali PA, Mirabella M
Clin. Immunol., 2005-11-07;118(1):77-82.
Species: Human
Sample Types: Cell Culture Supernates
-
Intracerebral transplantation of carotid body in rats with transient middle cerebral artery occlusion.
Authors: Yu G, Xu L, Hadman M, Hess DC, Borlongan CV
Brain Res., 2004-07-23;1015(1):50-6.
Species: Rat
Sample Types: Tissue Homogenates
-
Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo.
Authors: Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC, Fink DJ
Mol. Ther., 2004-07-01;10(1):67-75.
Species: Rat
Sample Types: Tissue Homogenates
-
AF5, a CNS cell line immortalized with an N-terminal fragment of SV40 large T: growth, differentiation, genetic stability, and gene expression.
Authors: Truckenmiller ME, Vawter MP, Zhang P, Conejero-Goldberg C, Dillon-Carter O, Morales N, Cheadle C, Becker KG, Freed WJ
Exp. Neurol., 2002-06-01;175(0):318.
Species: Rat
Sample Types: Cell Culture Supernates
-
Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson's disease using GDNF.
Authors: Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P
Exp. Neurol., 2000-07-01;164(1):15-24.
Species: Human
Sample Types: Tissue Homogenates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Human GDNF DuoSet ELISA
Average Rating: 5 (Based on 2 Reviews)
Have you used Human GDNF DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
We are using this kit to detect the release of GDNF in serum of animal after implant our cells