Human/Mouse E-Cadherin Antibody Summary
Asp155-Ile707
Accession # P12830
Applications
Please Note: Optimal dilutions should be determined by each laboratory for each application. General Protocols are available in the Technical Information section on our website.
Scientific Data
 View Larger
View Larger
Detection of Human and Mouse E‑Cadherin by Western Blot. Western blot shows lysates of A431 human epithelial carcinoma cell line, A549 human lung carcinoma cell line, HepG2 human hepatocellular carcinoma cell line, P19 mouse embryonal carcinoma cell line, and 4T1 mouse breast cancer cell line. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). A specific band was detected for E-Cadherin at approximately 110 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 1.
 View Larger
View Larger
Detection of E‑Cadherin in MCF‑7 Human Cell Line by Flow Cytometry. MCF-7 human breast cancer cell line was stained with Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648, filled histogram) or isotype control antibody (Catalog # AB-108-C, open histogram), followed by Allophycocyanin-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0108). View our protocol for Staining Membrane-associated Proteins.
 View Larger
View Larger
E‑Cadherin in Human Epidermoid Carcinoma Cells. E-Cadherin was detected in immersion fixed human epidermoid carcinoma cells using 10 µg/mL Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) for 3 hours at room temperature. Cells were stained with the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (red; Catalog # NL001) and counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
E‑Cadherin and SOX2 in BG01V Human Stem Cells. E-Cadherin and SOX2 were detected in BG01V human embryonic stem cells using 10 µg/mL Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) and 10 µg/mL Human/Mouse SOX2 Monoclonal Antibody (Catalog # MAB2018). Cells were incubated with primary antibodies for 3 hours at room temperature. Cells were stained for E-Cadherin using the NorthernLights™ 557-conjugated Anti-Goat IgG Secondary Antibody (green; Catalog # NL001) and for SOX2 using the NorthernLights 493-conjugated Anti-Mouse Secondary Antibody (red; Catalog # NL009). Cells were counterstained with DAPI (blue). View our protocol for Fluorescent ICC Staining of Cells on Coverslips.
 View Larger
View Larger
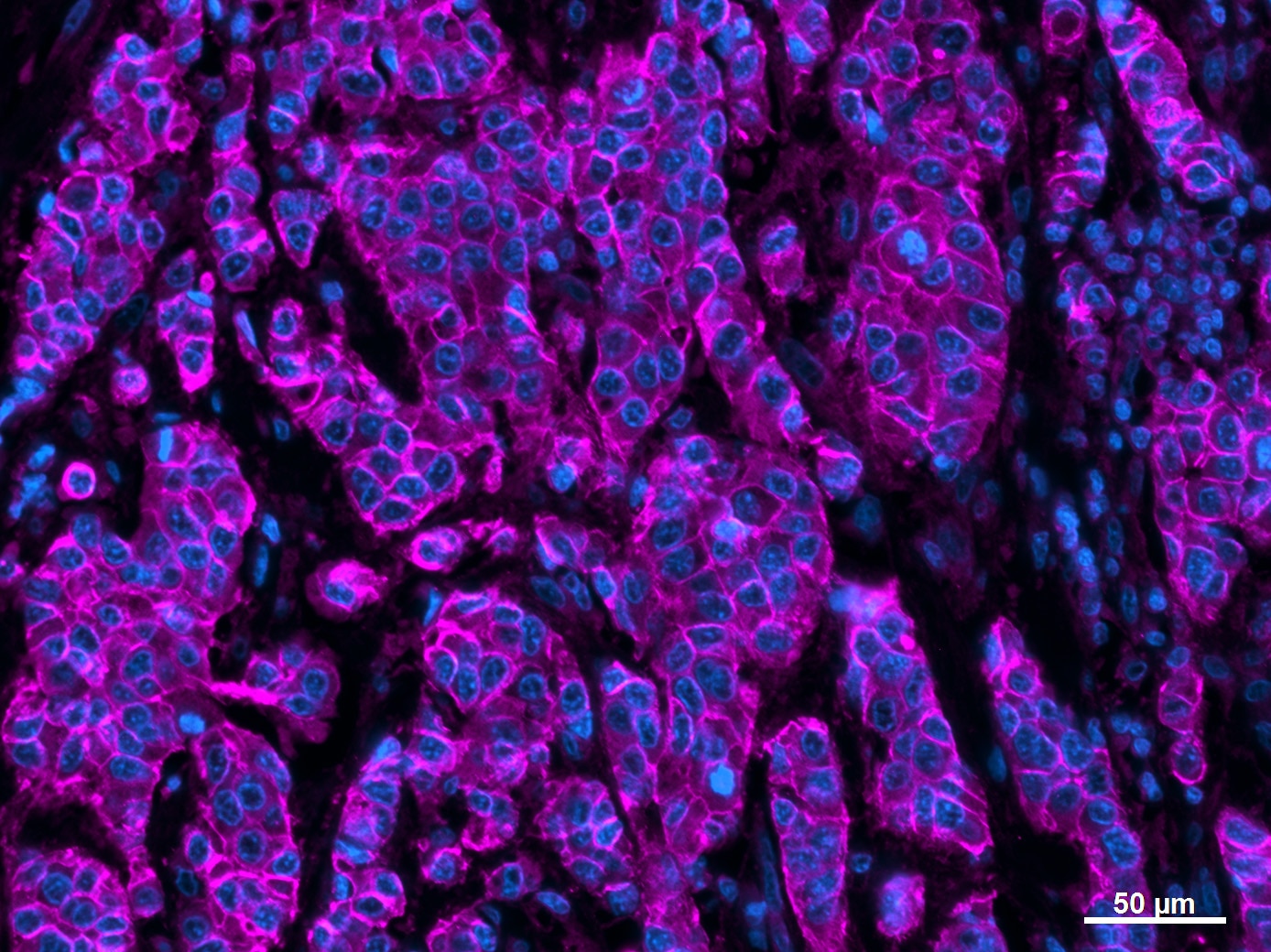
E‑Cadherin in Human Stomach. E-Cadherin was detected in immersion fixed paraffin-embedded sections of human stomach using Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) at 0.3 µg/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte™ HRP Polymer Antibody (Catalog # VC004). Tissue was stained using DAB (brown) and counterstained with hematoxylin (blue). Specific staining was localized to cell membrane and cytoplasm in gastric glands. View our protocol for IHC Staining with VisUCyte HRP Polymer Detection Reagents.
 View Larger
View Larger
Detection of Human and Mouse E‑Cadherin by Simple WesternTM. Simple Western lane view shows lysates of 4T1 mouse breast cancer cell line, P19 mouse embryonal carcinoma cell line, A431 human epithelial carcinoma cell line, and MCF-7 human breast cancer cell line, loaded at 0.2 mg/mL. A specific band was detected for E-Cadherin at approximately 128 kDa (as indicated) using 5 µg/mL of Goat Anti-Human/Mouse E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) followed by 1:50 dilution of HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF017). This experiment was conducted under reducing conditions and using the 12-230 kDa separation system.
 View Larger
View Larger
E-Cadherin in Human Stomach Using Dual RNAscope®ISH and IHC. E-Cadherin mRNA was detected in formalin-fixed paraffin-embedded tissue sections of human stomach probed with ACD RNAScope®Probe (Catalog # 311091) and stained using ACD RNAscope®2.5 HD Detection Reagents-Red (top image, Catalog # 32260). Adjacent tissue section was processed for immunohistochemistry using R&D Systems Goat Anti-Human E-Cadherin Antigen Affinity-purified Polyclonal Antibody (Catalog # AF648) at 0.3 ug/mL for 1 hour at room temperature followed by incubation with the Anti-Goat IgG VisUCyte HRP Polymer Antibody (R&D Systems, Catalog # VC004) and DAB chromogen (lower image, yellow-brown). Tissues were counterstained with hematoxylin (blue).
 View Larger
View Larger
Detection of Human E-Cadherin by Immunocytochemistry/Immunofluorescence Impact of H. pylori infection on ES cell-derived gastric organoids. (a) HE staining of ES-cell derived gastric organoids infected with H. pylori for 12 hours. Scale bar: 200 µm. (b) Immunostaining of gastric organoids infected with H. pylori for 12 hours. “No infection” indicates that the organoid was injected with bacteria-free Brucella Broth. HP, H. pylori; E-cad, E-cadherin. Scale bar: 10 µm. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/30374120), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Human E-Cadherin by Immunocytochemistry/Immunofluorescence Development and Characterization of an iPSC-Derived Fallopian Tube Organoid. (a) Schematic of factors involved in the differentiation of fallopian tube organoids. (b) Bright field image and H&E staining of FTE organoid at day 14. (c–e) Immunocytochemistry for FTE markers TUBB4A, FOXJ1 and PAX8 and epithelial marker CDH1 (E-Cadherin) at organoid culture day 14. (f) Immunocytochemistry for FTE markers PAX8, TUBB4A, OVGP1 and epithelial marker CDH1 at FTE organoid culture day 45, along with human fallopian tube tissue. (g) Immunocytochemistry for FTE markers TUBB4A and PAX8 at FTE organoid culture day 45. (h) Gene expression of fallopian tube markers OVGP1 (for 2 different primers), FOXJ1, TNFaIP2 and PAX8, as well as kidney markers SALL1 and FOXD1 at organoids culture day 45, human fallopian tube and kidney. The color matrix of the heat map represents the log2(Ratio) of each individual gene relative to its expression at the iPSC stage. Relative gene expression to iPSC stage (day 0) was calculated using delta delta Ct method and normalized to endogenous GAPDH level for 87iCTR-n3 iPSC line (i) H&E staining of FTE organoid at culture day 45 and day 180, and human fallopian tube tissue. Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/28878359), licensed under a CC-BY license. Not internally tested by R&D Systems.
 View Larger
View Larger
Detection of Mouse E-Cadherin by Immunohistochemistry Immunohistological visualization of engineered EcN strains in tissue sections.These sections are taken from proximal colons of mice receiving different bacteria. Sectioning protocol was designed to preserve mucus and luminal content. Sections were stained with fluorescently labeled antibodies: anti-E-cadherin (green), anti-Muc2 (red), and anti-LPS (blue). The first column shows bright-field images of the sections. The last column shows an overlay of all stains. The white dotted lines represent the boundary of the epithelium and mucus layers. The leftmost parts represent the epithelium, the center parts represent the mucus layers and the rightmost parts represent the lumen (scale bar = 100 μm). Image collected and cropped by CiteAb from the following publication (https://pubmed.ncbi.nlm.nih.gov/31811125), licensed under a CC-BY license. Not internally tested by R&D Systems.
Reconstitution Calculator
Preparation and Storage
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 6 months, -20 to -70 °C under sterile conditions after reconstitution.
Background: E-Cadherin
Epithelial (E)‑Cadherin (ECAD), also known as cell-CAM120/80 in the human, uvomorulin in the mouse, Arc-1 in the dog, and L-CAM in the chicken, is a member of the Cadherin family of cell adhesion molecules. Cadherins are calcium-dependent transmembrane proteins which bind to one another in a homophilic manner. On their cytoplasmic side, they associate with the three catenins, alpha, beta, and gamma (plakoglobin). This association links the cadherin protein to the cytoskeleton. Without association with the catenins, the cadherins are non-adhesive. Cadherins play a role in development, specifically in tissue formation. They may also help to maintain tissue architecture in the adult. E‑Cadherin may also play a role in tumor development, as loss of E‑Cadherin has been associated with tumor invasiveness. E‑Cadherin is a classical cadherin molecule. Classical cadherins consist of a large extracellular domain which contains DXD and DXNDN repeats responsible for mediating calcium‑dependent adhesion, a single-pass transmembrane domain, and a short carboxy-terminal cytoplasmic domain responsible for interacting with the catenins. E‑Cadherin contains five extracellular calcium‑binding domains of approximately 110 amino acids each.
- Bussemakers, M.J.G. et al. (1993) Mol. Biol. Reports 17:123.
- Overduin, M. et al. (1995) Science 267:386.
- Takeichi, M. (1991) Science 251:1451.
Product Datasheets
Citations for Human/Mouse E-Cadherin Antibody
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
78
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
An Organoid-Based Preclinical Model of Human Gastric Cancer
Authors: NG Steele, J Chakrabart, J Wang, J Biesiada, L Holokai, J Chang, LM Nowacki, J Hawkins, M Mahe, N Sundaram, N Shroyer, M Medvedovic, M Helmrath, S Ahmad, Y Zavros
Cell Mol Gastroenterol Hepatol, 2018-09-20;7(1):161-184.
-
Essential Functions of Glycans in Human Epithelia Dissected by a CRISPR-Cas9-Engineered Human Organotypic Skin Model
Authors: Sally Dabelsteen, Emil M.H. Pallesen, Irina N. Marinova, Mathias I. Nielsen, Maria Adamopoulou, Troels B. Rømer et al.
Developmental Cell
-
Directed differentiation of human pluripotent stem cells into epidermal stem and progenitor cells.
Authors: Sonya Ruiz-Torres, Paul F. Lambert, Kathryn A. Wikenheiser-Brokamp, Susanne I. Wells
Molecular Biology Reports
-
Keeping Candida commensal - How lactobacilli antagonize pathogenicity of Candida albicans in an in vitro gut model
Authors: Graf K, Last A, Gratz R et al.
Dis Model Mech
-
Retinoic acid receptor gamma activation promotes differentiation of human induced pluripotent stem cells into esophageal epithelium
Authors: Yasufumi Koterazawa, Michiyo Koyanagi-Aoi, Keiichiro Uehara, Yoshihiro Kakeji, Takashi Aoi
Journal of Gastroenterology
-
Inhibition of TGF-beta 1 Signaling by IL-15: A Novel Role for IL-15 in the Control of Renal Epithelial-Mesenchymal Transition: IL-15 Counteracts TGF-beta 1-Induced EMT in Renal Fibrosis
Authors: Aurore Devocelle, Lola Lecru, Hélène François, Christophe Desterke, Cindy Gallerne, Pierre Eid et al.
International Journal of Cell Biology
-
Transplanted organoids empower human preclinical assessment of drug candidate for the clinic
Authors: AD Westerling, EM Fast, TW Soare, S Venkatacha, M DeRan, AB Fanelli, S Kyrychenko, H Hoang, GM Corriea, W Zhang, M Yu, M Daniels, G Malojcic, XR Pan-Zhou, MW Ledeboer, JC Harmange, M Emani, TT Tibbitts, JF Reilly, P Mundel
Science Advances, 2022-07-06;8(27):eabj5633.
-
Single cell analysis of endometriosis reveals a coordinated transcriptional program driving immunotolerance and angiogenesis across eutopic and ectopic tissues
Authors: Tan Y, Flynn W, Sivajothi S Et al.
Nat Cell Biol
-
Human mastadenovirus-B (HAdV-B)-specific E3-CR1 beta and E3-CR1 gamma glycoproteins interact with each other and localize at the plasma membrane of non-polarized airway epithelial cells
Authors: Poornima Kotha Lakshmi Narayan, Adriana E. Kajon
Virology
-
A Tgfbr1/Snai1-dependent developmental module at the core of vertebrate axial elongation
Authors: André Dias, Anastasiia Lozovska, Filip J Wymeersch, Ana Nóvoa, Anahi Binagui-Casas, Daniel Sobral et al.
eLife
-
Generation of multi-cellular human liver organoids from pluripotent stem cells
Authors: Wendy L. Thompson, Takanori Takebe
Methods in Cell Biology
-
Generation of human colonic organoids from human pluripotent stem cells
Authors: Abdelkader Daoud, Jorge O. Múnera
Methods in Cell Biology
-
Collaborating genomic, transcriptomic and microbiomic alterations lead to canine extreme intestinal polyposis
Authors: Jin Wang, Tianfang Wang, Micah A. Bishop, John F. Edwards, Hang Yin, Stephen Dalton et al.
Oncotarget
-
BATF3 Protects Against Metabolic Syndrome and Maintains Intestinal Epithelial Homeostasis
Authors: Hussein Hamade, Jasmine T. Stamps, Dalton T. Stamps, Shyam K. More, Lisa S. Thomas, Anna Y. Blackwood et al.
Frontiers in Immunology
-
A combined human gastruloid model of cardiogenesis and neurogenesis
Authors: Olmsted ZT, Paluh JL.
iScience
-
Development of a Personalized Intestinal Fibrosis Model Using Human Intestinal Organoids Derived From Induced Pluripotent Stem Cells
Authors: Hannah Q Estrada, Shachi Patel, Shervin Rabizadeh, David Casero, Stephan R Targan, Robert J Barrett
Inflammatory Bowel Diseases
-
HPV Strain Predicts Severity of Juvenile-Onset Recurrent Respiratory Papillomatosis with Implications for Disease Screening
Authors: Mary C. Bedard, Alessandro de Alarcon, Yann-Fuu Kou, David Lee, Alexandra Sestito, Angela L. Duggins et al.
Cancers (Basel)
-
CDX2-induced intestinal metaplasia in human gastric organoids derived from induced pluripotent stem cells
Authors: Takahiro Koide, Michiyo Koyanagi-Aoi, Keiichiro Uehara, Yoshihiro Kakeji, Takashi Aoi
iScience
-
BMAL1 Knockdown Leans Epithelial–Mesenchymal Balance toward Epithelial Properties and Decreases the Chemoresistance of Colon Carcinoma Cells
Authors: Yuan Zhang, Aurore Devocelle, Christophe Desterke, Lucas Eduardo Botelho de Souza, Éva Hadadi, Hervé Acloque et al.
International Journal of Molecular Sciences
-
Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis
Authors: Wei Rao, Shan Wang, Marcin Duleba, Suchan Niroula, Kristina Goller, Jingzhong Xie et al.
Cell
-
MMP20 Cleaves E-Cadherin and Influences Ameloblast Development
Authors: John D. Bartlett, Yasuo Yamakoshi, James P. Simmer, Antonio Nanci, Charles E. Smith
Cells Tissues Organs
-
DR3 regulates intestinal epithelial homeostasis and regeneration after intestinal barrier injury
Authors: Y Shimodaira, SK More, H Hamade, AY Blackwood, JP Abraham, LS Thomas, JH Miller, DT Stamps, SL Castanon, N Jacob, CW Y Ha, S Devkota, DQ Shih, SR Targan, KS Michelsen
Cellular and Molecular Gastroenterology and Hepatology, 2023-04-01;0(0):.
-
Human Pluripotent Stem Cell-Derived Multipotent Vascular Progenitors of the Mesothelium Lineage Have Utility in Tissue Engineering and Repair
Authors: T Colunga, M Hayworth, S Kre beta, DM Reynolds, L Chen, KL Nazor, J Baur, AM Singh, JF Loring, M Metzger, S Dalton
Cell Rep, 2019-03-05;26(10):2566-2579.e10.
-
Malfunction of airway basal stem cells plays a crucial role in pathophysiology of tracheobronchopathia osteoplastica
Authors: Y Hong, S Shan, Y Gu, H Huang, Q Zhang, Y Han, Y Dong, Z Liu, M Huang, T Ren
Nature Communications, 2022-03-14;13(1):1309.
-
Cytochrome P450 expression, induction and activity in human induced pluripotent stem cell-derived intestinal organoids and comparison with primary human intestinal epithelial cells and Caco-2 cells
Authors: Aafke W. F. Janssen, Loes P. M. Duivenvoorde, Deborah Rijkers, Rosalie Nijssen, Ad A. C. M. Peijnenburg, Meike van der Zande et al.
Archives of Toxicology
-
Integrating collecting systems in kidney organoids through fusion of distal nephron to ureteric bud
Authors: Shi, M;Crouse, B;Sundaram, N;Shakked, NP;Ester, L;Zhang, W;Janakiram, V;Kopan, R;Helmrath, MA;Bonventre, JV;McCracken, KW;
bioRxiv : the preprint server for biology
Species: Human
Sample Types: Organoid
Applications: Immunocytochemistry -
Chemically-defined and scalable culture system for intestinal stem cells derived from human intestinal organoids
Authors: Kwon, O;Lee, H;Jung, J;Son, YS;Jeon, S;Yoo, WD;Son, N;Jung, KB;Choi, E;Lee, IC;Kwon, HJ;Kim, C;Lee, MO;Cho, HS;Kim, DS;Son, MY;
Nature communications
Species: Human
Sample Types: Whole Cells
Applications: Immunocytochemistry/Immunofluorescence -
Gasdermin D promotes hyperinflammation and immunopathology during severe influenza A virus infection
Authors: Rosli, S;Harpur, CM;Lam, M;West, AC;Hodges, C;Mansell, A;Lawlor, KE;Tate, MD;
Cell death & disease
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
The minichromosome maintenance complex drives esophageal basal zone hyperplasia
Authors: Rochman, ME;Rochman, Y;Caldwell, JM;Mack, L;Besse, JA;Manes, NP;Yoon, SH;Shoda, T;Nita-Lazar, A;Rothenberg, M;
JCI insight
Species: Human, Transgenic Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
DR3 regulates intestinal epithelial homeostasis and regeneration after intestinal barrier injury
Authors: Y Shimodaira, SK More, H Hamade, AY Blackwood, JP Abraham, LS Thomas, JH Miller, DT Stamps, SL Castanon, N Jacob, CW Y Ha, S Devkota, DQ Shih, SR Targan, KS Michelsen
Cellular and Molecular Gastroenterology and Hepatology, 2023-04-01;0(0):.
-
Pre-hyperglycemia immune cell trafficking underlies subclinical diabetic cataractogenesis
Authors: E Ranaei Pir, Y Zhang, A Barakat, M Naseri, C Russmann, A Hafezi-Mog
Journal of Biomedical Science, 2023-01-24;30(1):6.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
A combined human gastruloid model of cardiogenesis and neurogenesis
Authors: Olmsted ZT, Paluh JL.
iScience
-
Epithelial-derived factors induce muscularis mucosa of human induced pluripotent stem cell-derived gastric organoids
Authors: K Uehara, M Koyanagi-A, T Koide, T Itoh, T Aoi
Stem Cell Reports, 2022-03-03;0(0):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Human iPSC-derived fallopian tube organoids with BRCA1 mutation recapitulate early-stage carcinogenesis
Authors: N Yucer, R Ahdoot, MJ Workman, AH Laperle, MS Recouvreux, K Kurowski, DJ Naboulsi, V Liang, Y Qu, JT Plummer, SA Gayther, S Orsulic, BY Karlan, CN Svendsen
Cell Reports, 2021-12-28;37(13):110146.
Species: Human
Sample Types: Organoid, Whole Cells
Applications: ICC, IHC -
Functional human gastrointestinal organoids can be engineered from three primary germ layers derived separately from pluripotent stem cells
Authors: AK Eicher, DO Kechele, N Sundaram, HM Berns, HM Poling, LE Haines, JG Sanchez, K Kishimoto, M Krishnamur, L Han, AM Zorn, MA Helmrath, JM Wells
Cell Stem Cell, 2021-12-01;0(0):.
Species: Human, Mouse
Sample Types: Whole Tissue
Applications: IHC -
A Wnt-mediated phenotype switch along the epithelial-mesenchymal axis defines resistance and invasion downstream of ionising radiation in oral squamous cell carcinoma
Authors: F Zolghadr, N Tse, D Loka, G Joun, S Meppat, V Wan, H Zoellner, M Xaymardan, CS Farah, JG Lyons, E Hau, E Patrick, N Seyedasli
British Journal of Cancer, 2021-03-30;0(0):.
Species: Human
Sample Types: Cell Lysates, Organoid, Whole Cells
Applications: Flow Cytometry, IHC, Western Blot -
Induced organoids derived from patients with ulcerative colitis recapitulate colitic reactivity
Authors: SK Sarvestani, S Signs, B Hu, Y Yeu, H Feng, Y Ni, DR Hill, RC Fisher, S Ferrandon, RK DeHaan, J Stiene, M Cruise, TH Hwang, X Shen, JR Spence, EH Huang
Nature Communications, 2021-01-11;12(1):262.
Species: Human
Sample Types: Organoid
Applications: IHC -
Patient-Derived Organotypic Epithelial Rafts Model Phenotypes in Juvenile-Onset Recurrent Respiratory Papillomatosis
Authors: MC Bedard, MG Brusadelli, A Carlile, S Ruiz-Torre, H Lodin, D Lee, M Kofron, PF Lambert, A Lane, N Ameziane, EM Bahassi, KA Wikenheise, A de Alarcon, DF Smith, SI Wells
Viruses, 2021-01-06;13(1):.
Species: Human
Sample Types: Whole Tissue
Applications: IHC -
Modeling Intestinal Epithelial Response to Interferon-&gamma in Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids
Authors: MJ Workman, E Troisi, SR Targan, CN Svendsen, RJ Barrett
International Journal of Molecular Sciences, 2020-12-30;22(1):.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Claudin-18 Loss Alters Transcellular Chloride Flux but not Tight Junction Ion Selectivity in Gastric Epithelial Cells
Authors: TJ Caron, KE Scott, N Sinha, S Muthupalan, M Baqai, LH Ang, Y Li, JR Turner, JG Fox, SJ Hagen
Cell Mol Gastroenterol Hepatol, 2020-10-16;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC -
Repair of acute liver damage with immune evasive hESC derived hepato-blasts
Authors: J Liu, T Pan, Y Chen, Y Liu, F Yang, Q Chen, N Abbas, M Zhong, Q Zhang, Y Xu, YX Li
Stem Cell Res, 2020-09-26;49(0):102010.
Species: Human, Mouse
Sample Types: Whole Cells, Whole Tissue
Applications: ICC, IHC -
Modulation of glycosyltransferase ST6Gal-I in gastric cancer-derived organoids disrupts homeostatic epithelial cell turnover
Authors: KL Alexander, CA Serrano, A Chakrabort, M Nearing, LN Council, A Riquelme, M Garrido, SL Bellis, LE Smythies, PD Smith
J. Biol. Chem., 2020-08-06;0(0):.
Species: Human
Sample Types: Organoid
Applications: ICC, IHC -
Engineered E. coli Nissle 1917 for the delivery of matrix-tethered therapeutic domains to the gut
Authors: P Praveschot, AM Duraj-That, I Gelfat, F Bahl, DB Chou, NS Joshi
Nat Commun, 2019-12-06;10(1):5580.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
miR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo
Authors: M Kiener, L Chen, M Krebs, J Grosjean, I Klima, C Kalogirou, H Riedmiller, B Kneitz, GN Thalmann, E Snaar-Jaga, M Spahn, M Kruithof-d, E Zoni
BMC Cancer, 2019-06-25;19(1):627.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Human Pluripotent Stem Cell-Derived Kidney Model for Nephrotoxicity Studies.
Authors: Bajaj P, Rodrigues A, Steppan C, Engle S, Mathialagan S, Schroeter T
Drug Metab Dispos, 2018-08-31;46(11):1703-1711.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Acoustic Tweezing Cytometry Induces Rapid Initiation of Human Embryonic Stem Cell Differentiation
Authors: T Topal, X Hong, X Xue, Z Fan, N Kanetkar, JT Nguyen, J Fu, CX Deng, PH Krebsbach
Sci Rep, 2018-08-28;8(1):12977.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
IDENTIFICATION OF PIPERAZINYLBENZENESULFONAMIDES AS NEW INHIBITORS OF CLAUDIN-1 TRAFFICKING AND HEPATITIS C VIRUS ENTRY
Authors: L Riva, OR Song, J Prentoe, F Helle, L L'homme, CH Gattolliat, A Vandeputte, L Fénéant, S Belouzard, TF Baumert, T Asselah, J Bukh, P Brodin, L Cocquerel, Y Rouillé, J Dubuisson
J. Virol., 2018-04-27;0(0):.
Species: Human
Sample Types: Whole Cells
Applications: Flow Cytometry -
A novel transforming growth factor beta-induced long noncoding RNA promotes an inflammatory microenvironment in human intrahepatic cholangiocarcinoma
Authors: A Merdrignac, G Angenard, C Allain, K Petitjean, D Bergeat, P Bellaud, A Fautrel, B Turlin, B Clément, S Dooley, L Sulpice, K Boudjema, C Coulouarn
Hepatol Commun, 2018-01-30;2(3):254-269.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Enhanced Utilization of Induced Pluripotent Stem Cell-Derived Human Intestinal Organoids Using Microengineered Chips.
Authors: Michael J Workman, John P Gleeson, Elissa J Troisi, Hannah Q Estrada, S Jordan Kerns, Christopher D Hinojosa, Geraldine A Hamilton, Stephan R Targan, Clive N Svendsen, Robert J Barrett
Cellular and Molecular Gastroenterology and Hepatology, 2017-12-29;0(0):2352-345X.
Species: Human
Sample Types:
Applications: ICC -
Placenta-specific protein 1 promotes cell proliferation and invasion in non-small cell lung cancer
Authors: L Yang, TQ Zha, X He, L Chen, Q Zhu, WB Wu, FQ Nie, Q Wang, CS Zang, ML Zhang, J He, W Li, W Jiang, KH Lu
Oncol. Rep., 2017-11-09;39(1):53-60.
Species: Human
Sample Types: Cell Lysates
Applications: Western Blot -
Directed Differentiation of Human Induced Pluripotent Stem Cells into Fallopian Tube Epithelium
Authors: N Yucer, M Holzapfel, T Jenkins Vo, L Lenaeus, L Ornelas, A Laury, D Sareen, R Barrett, BY Karlan, CN Svendsen
Sci Rep, 2017-09-06;7(1):10741.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
miR-151a induces partial EMT by regulating E-cadherin in NSCLC cells
Authors: I Daugaard, KJ Sanders, A Idica, K Vittayaruk, M Hamdorf, JD Krog, R Chow, D Jury, LL Hansen, H Hager, P Lamy, CL Choi, D Agalliu, DG Zisoulis, IM Pedersen
Oncogenesis, 2017-07-31;6(7):e366.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Surface Topography Guides Morphology and Spatial Patterning of Induced Pluripotent Stem Cell Colonies
Authors: G Abagnale, A Sechi, M Steger, Q Zhou, CC Kuo, G Aydin, C Schalla, G Müller-New, M Zenke, IG Costa, P van Rijn, A Gillner, W Wagner
Stem Cell Reports, 2017-07-27;9(2):654-666.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection
Authors: P Treerat, O Prince, A Cruz-Lagun, M Muñoz-Torr, MA Salazar-Le, M Selman, B Fallert-Ju, TA Reinhardt, JF Alcorn, D Kaushal, J Zuñiga, J Rangel-Mor, JK Kolls, SA Khader
Mucosal Immunol, 2017-03-01;0(0):.
Species: Primate - Macaca mulatta (Rhesus Macaque)
Sample Types: Whole Tissue
Applications: IHC -
A process engineering approach to increase organoid yield
Authors: N Arora, J Imran Also, JW Guggenheim, M Mak, J Munera, JM Wells, RD Kamm, HH Asada, SY Shvartsman, LG Griffith
Development, 2017-02-07;144(6):1128-1136.
Species: Human
Sample Types: Spheroid
Applications: IHC -
Wnt/?-catenin promotes gastric fundus specification in mice and humans
Authors: KW McCracken, E Aihara, B Martin, CM Crawford, T Broda, J Treguier, X Zhang, JM Shannon, MH Montrose, JM Wells
Nature, 2017-01-04;0(0):.
Species: Mouse
Sample Types: Whole Tissue
Applications: IHC-P -
Canine spontaneous head and neck squamous cell carcinomas represent their human counterparts at the molecular level.
Authors: Liu D, Xiong H, Ellis A, Northrup N, Dobbin K, Shin D, Zhao S
PLoS Genet, 2015-06-01;11(6):e1005277.
Species: Canine
Sample Types: Whole Cells
Applications: IHC-P -
Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer.
Authors: Liu D, Xiong H, Ellis A, Northrup N, Rodriguez C, O'Regan R, Dalton S, Zhao S
Cancer Res, 2014-07-31;74(18):5045-56.
Species: Canine
Sample Types: Whole Tissue
Applications: IHC-P -
Novel human renal proximal tubular cell line for the production of complex proteins.
Authors: Fliedl L, Manhart G, Kast F, Katinger H, Kunert R, Grillari J, Wieser M, Grillari-Voglauer R
J Biotechnol, 2014-02-16;176(0):29-39.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Penton-dodecahedral particles trigger opening of intercellular junctions and facilitate viral spread during adenovirus serotype 3 infection of epithelial cells.
Authors: Lu Z, Wang H, Zhang Y, Cao H, Li Z, Fender P, Lieber A
PLoS Pathog, 2013-10-31;9(10):e1003718.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Interleukin-15 plays a central role in human kidney physiology and cancer through the gamma c signaling pathway.
Authors: Giron-Michel J, Azzi S, Khawam K, Mortier E, Caignard A, Devocelle A, Ferrini S, Croce M, Francois H, Lecru L, Charpentier B, Chouaib S, Azzarone B, Eid P
PLoS ONE, 2012-02-21;7(2):e31624.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Snail involves in the transforming growth factor beta1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells.
Authors: Li H, Wang H, Wang F, Gu Q, Xu X
PLoS ONE, 2011-08-10;6(8):e23322.
Species: Human
Sample Types: Cell Lysates, Whole Cells
Applications: ICC, Western Blot -
Tissue engineering of oral dysplasia.
Authors: Gaballah K, Costea DE, Hills A, Gollin SM, Harrison P, Partridge M
J. Pathol., 2008-07-01;215(3):280-9.
Species: Human
Sample Types: Whole Cells
Applications: ICC -
Downregulation of Rap1GAP contributes to Ras transformation.
Authors: Tsygankova OM, Prendergast GV, Puttaswamy K, Wang Y, Feldman MD, Wang H, Brose MS, Meinkoth JL
Mol. Cell. Biol., 2007-07-23;27(19):6647-58.
Species: Human, Ovine
Sample Types: Cell Lysates
Applications: Western Blot -
Requirement for intercellular adhesion molecule 1 and caveolae in invasion of human oral epithelial cells by Porphyromonas gingivalis.
Authors: Tamai R, Asai Y, Ogawa T
Infect. Immun., 2005-10-01;73(10):6290-8.
Species: Human
Sample Types: Whole Cells
Applications: Neutralization -
Methyl Donors Reduce Cell Proliferation by Diminishing Erk-Signaling and NFkB Levels, While Increasing E-Cadherin Expression in Panc-1 Cell Line
Authors: Eva Kiss, Gertrud Forika, Magdolna Dank, Tibor Krenacs, Zsuzsanna Nemeth
International Journal of Molecular Sciences
-
Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis
Authors: Cui W, Sathyanarayan A, Lopresti M et al.
Autophagy
-
Engineering human hepato-biliary-pancreatic organoids from pluripotent stem cells
Authors: Hiroyuki Koike, Kentaro Iwasawa, Rie Ouchi, Mari Maezawa, Masaki Kimura, Asuka Kodaka et al.
Nature Protocols
-
Neonatal Injury Increases Gut Permeability by Epigenetically Suppressing E-Cadherin in Adulthood
Authors: KT Kline, H Lian, XS Zhong, X Luo, JH Winston, Y Cong, TC Savidge, RH Dashwood, DW Powell, Q Li
J. Immunol., 2019-12-30;0(0):.
-
Modelling human development and disease in pluripotent stem-cell-derived gastric organoids
Authors: Kyle W. McCracken, Emily M. Catá, Calyn M. Crawford, Katie L. Sinagoga, Michael Schumacher, Briana E. Rockich et al.
Nature
-
Generation of human elongating multi-lineage organized cardiac gastruloids
Authors: Zachary T. Olmsted, Maria Belen Paredes-Espinosa, Janet L. Paluh
STAR Protocols
-
Role of BCL9L in transforming growth factor-beta (TGF-beta )-induced epithelial-to-mesenchymal-transition (EMT) and metastasis of pancreatic cancer
Authors: Giuseppina Sannino, Nicole Armbruster, Mona Bodenhöfer, Ursula Haerle, Diana Behrens, Malte Buchholz et al.
Oncotarget
-
Evaluating the origin and virulence of a Helicobacter pylori cagA-positive strain isolated from a non-human primate
Authors: Kana Hashi, Chihiro Imai, Koji Yahara, Kamrunnesa Tahmina, Takeru Hayashi, Takeshi Azuma et al.
Scientific Reports
-
Multipotent Vascular Progenitor Cells of the Mesothelium Lineage Generated from Human Pluripotent Stem Cells
Authors: Luoman Chen, Stephen Dalton
STAR Protocols
-
Induced hepatic stem cells are suitable for human hepatocyte production
Authors: Yoshiki Nakashima, Chika Miyagi-Shiohira, Issei Saitoh, Masami Watanabe, Masayuki Matsushita, Masayoshi Tsukahara et al.
iScience
-
Terbium-based time-gated Forster resonance energy transfer imaging for evaluating protein-protein interactions on cell membranes
Authors: Linden S, Singh MK, Wegner KD et al.
Dalton Trans.
-
A Dual Array-Based Approach to Assess the Abundance and Posttranslational Modification State of Signaling Proteins
Authors: Katrin Luckert, Taranjit S. Gujral, Marina Chan, Mark Sevecka, Thomas O. Joos, Peter K. Sorger et al.
Science Signaling
-
Activatable Zymography Probes Enable In Situ Localization of Protease Dysregulation in Cancer
Authors: Ava P. Soleimany, Jesse D. Kirkpatrick, Susan Su, Jaideep S. Dudani, Qian Zhong, Ahmet Bekdemir et al.
Cancer Research
FAQs
No product specific FAQs exist for this product, however you may
View all Antibody FAQsReviews for Human/Mouse E-Cadherin Antibody
Average Rating: 4 (Based on 6 Reviews)
Have you used Human/Mouse E-Cadherin Antibody?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
IHC staining for CDH1 was performed on human colon cancer tissue
The antibody did not give any specific staining on mouse paraffin sections of choroid plexus, Epitope retrieval: Sodium citrate
***Bio-Techne Response: Thank you for reviewing our product. We are sorry to hear that this antibody did not perform as expected. We have been in touch with the customer to resolve this issue according to our Product Guarantee and to the customer’s satisfaction.***
Antigen retrieval: 10mM Citrate pH6
Antibody dilution: 1:400
Secondary antibody: Alexa 647 donkey anti-goat secondary antibody
Antigen retrieval: 10mM Citrate buffer pH6.
Antibody dilution: 1:400
Secondary antibody: CY3 conjugated donkey anti-goat secondary antibody @ 1:300