Immunology News: Researchers Demonstrate the Importance of Treg Subset Selection and sLeX Expression for Human Treg Immunotherapy
Regulatory T cells (Tregs) are a unique subset of CD4+ T cells with immunosuppressive properties. As a result, they are currently being investigated as a cellular therapeutic for the treatment of autoimmune diseases and long-term patient and graft survival following solid organ transplantation, and allogeneic hematopoietic stem cell transplantation. Studies in animal models suggest that the success of Treg cell therapy relies on the presence of a high number of Tregs in order to suppress the activities of effector T cells. Since the frequency of Tregs in the peripheral blood is low, Treg cell therapy typically requires that Tregs be isolated and expanded in vitro and then infused back into the patient. Complicating these procedures is the fact that several Treg subsets have been identified that vary in terms of their suppressive capacities and their abilities to be expanded in vitro while still maintaining purity. Additionally, Tregs display functional plasticity and can transition into pro-inflammatory FoxP3+ cells that secrete IL-17 and/or IFN-gamma or FoxP3- cells that lack suppressive activity. As a result, there has not only been variations in the procedures being used to isolate and expand Tregs for cell therapy, but there has also been a lack of consistency in the purity of the Treg populations being used, along with an incomplete understanding of how Treg populations may differ functionally. These issues may explain why Treg cell therapies have not been more successful thus far.
In an article published in Scientific Reports in January 2018, Donnelly, C. et al. outlined the characteristics of a Treg population that would be ideally suited for cell therapy. This population of Tregs should not only be highly suppressive and have a high capacity to be expanded in vitro, but these cells also need to maintain their purity and lineage fidelity during the expansion procedure and be capable of migrating to inflammatory sites. Since multiple, functionally distinct Treg populations have been identified including naïve Tregs, HLA-DR+ effector memory Tregs, and HLA-DR- memory Tregs, which differ phenotypically and functionally, Donnelly, C. et al. attempted to determine which of these Treg subpopulations would be most suitable for cell therapy based on the criteria that they outlined.
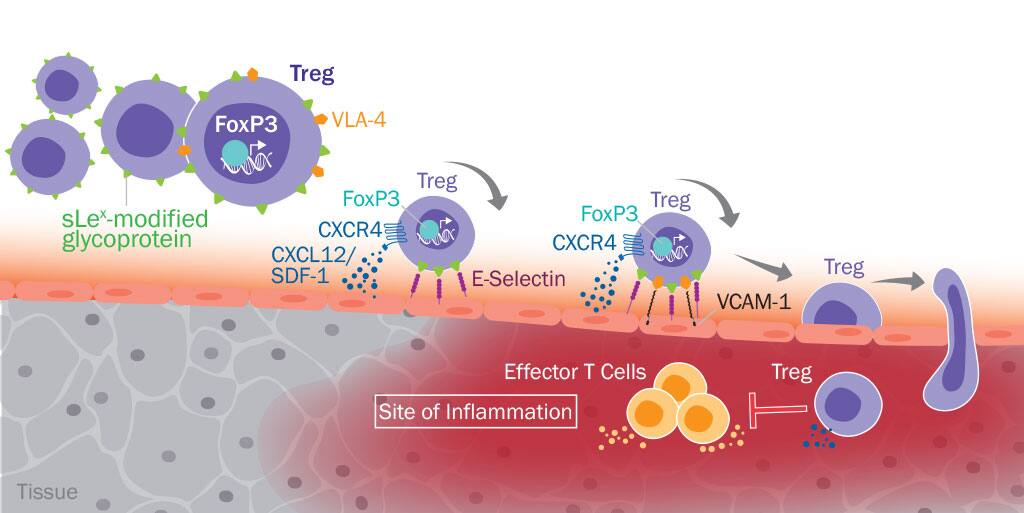
Using FACS-based sorting to isolate CD45RA+CD25highCD27low naïve Tregs, CD45RA-CD25highCD27lowHLA-DR+ effector memory Tregs, and CD45RA-CD25highCD27lowHLA-DR- memory Tregs from the blood of healthy donors, the authors showed that when each of these Treg subpopulations was co-cultured with CFSE-labeled CD4+CD25-CD45RA- T cells at a suboptimal ratio under different stimulation conditions, HLA-DR+ effector memory Tregs were consistently the most suppressive Treg population, while naïve Tregs were routinely the least suppressive of the three subtypes. Additionally, the authors showed that HLA-DR+ effector memory Tregs expressed the highest levels of sialyl-Lewis X tetra-saccharide (sLeX). sLeX is a glycan motif expressed on glycoproteins and glycolipids on the surface of lymphocytes that serves as an indicator of E-Selectin binding, which is critical for lymphocyte migration into inflammatory sites. Naïve Tregs expressed the lowest levels of sLeX, suggesting that these cells would likely display poor tissue homing in vivo. Upon further investigation, the authors found that naïve Tregs still expressed high levels of CD162 (PSGL1), CD44, and CD43, the cell surface proteins that would display sLeX glycosylation, but they expressed low levels of alpha-(1,3)-fucosyltransferase VII (FUT7) and high levels of fucosidase 1 (FUC1). FUT7 would contribute to the addition of the specific fucose linkage required for generating sLeX, while FUC1 would be involved in cleaving the fucose from the cell surface. As expected based on sLeX expression levels, naïve Tregs displayed the lowest capacity to adhere to TNF-alpha-stimulated E-Selectin+ endothelial cells and had the lowest static transendothelial migration (TEM) potential, while HLA-DR+ memory Tregs had the highest E-Selectin binding capacity and the most favorable transmigration potential. According to these results, it seemed that HLA-DR+ memory Tregs would be the most suitable for cell therapy as they exhibited the strongest suppressive capacity and had the highest potential to migrate to sites of inflammation. However, when each of these subpopulations was expanded in vitro, HLA-DR+ memory Tregs exhibited less than a 10-fold expansion, while naïve Tregs exhibited more than a 200-fold expansion. The expanded naïve Tregs also retained high levels of purity as assessed by sustained FoxP3 expression and a lack of cells co-expressing FoxP3 and IL-17 or IFN-gamma. In contrast, only approximately 50% of the expanded HLA-DRlow effector memory Tregs expressed FoxP3, indicating that these cells failed to maintain lineage fidelity or were contaminated with non-regulatory T effector cells. Additionally, along with the superior expansion potential and lineage fidelity associated with the in vitro expanded naïve Tregs, the authors unexpectedly found that these cells displayed a significant increase in their suppressive capacity relative to the freshly isolated naïve Tregs that they had originally tested.
Recognizing that several characteristics of the expanded naïve Treg population seemed to suggest that these cells would be ideal for cell therapy, the authors looked at the migration potential of these cells. They found that the expanded naïve Treg population displayed an increased ability to undergo TEM due to a substantial increase in the expression of CXCR4 and maintenance of high levels of VLA-4 expression. Unfortunately, however, the cells only had a minor increase in sLeX expression compared to the freshly isolated naïve Tregs. Since the low level of sLeX expression on these cells would reduce their efficacy as a cellular therapeutic, the authors asked whether in vitro treatment of the cells with FUT7 could increase sLeX expression and thereby improve their ability to home to inflamed tissues. Following treatment of the expanded naïve Tregs with purified, recombinant FUT7 and GDP-fucose, the authors saw a significant increase in sLeX expression compared to buffer-treated cells, which led to a striking increase in the ability of the cells to adhere to E-Selectin+ endothelial cells. Notably, they also observed no change in the suppressive capacity of the cells following FUT7 treatment. Using a xenogeneic mouse model of acute graft-versus-host disease (aGVHD), the authors subsequently showed that injection of the expanded naïve Tregs treated with FUT7 led to preferential homing of the cells to tissue expressing E-Selectin, as well as a significant reduction in disease severity when suboptimal numbers of the cells were injected. As a result of these findings, the authors have identified a new method to attempt to optimize human Treg immunotherapy, which could lead to greater clinical success.
Additional resources related to this topic:
Mechanisms of Regulatory T Cell-Mediated Suppression Interactive Pathway