Mouse VEGF DuoSet ELISA Summary
* Provided that the recommended microplates, buffers, diluents, substrates and solutions are used, and the assay is run as summarized in the Assay Procedure provided.
This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant mouse VEGF. The suggested diluent is suitable for the analysis of most cell culture supernate samples. Diluents for complex matrices, such as serum and plasma, should be evaluated prior to use in this DuoSet.
Product Features
- Optimized capture and detection antibody pairings with recommended concentrations save lengthy development time
- Development protocols are provided to guide further assay optimization
- Assay can be customized to your specific needs
- Economical alternative to complete kits
Kit Content
- Capture Antibody
- Detection Antibody
- Recombinant Standard
- Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP)
Other Reagents Required
DuoSet Ancillary Reagent Kit 2 (5 plates): (Catalog # DY008) containing 96 well microplates, plate sealers, substrate solution, stop solution, plate coating buffer (PBS), wash buffer, and Reagent Diluent Concentrate 2.
The components listed above may be purchased separately:
PBS: (Catalog # DY006), or 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2 - 7.4, 0.2 µm filtered
Wash Buffer: (Catalog # WA126), or 0.05% Tween® 20 in PBS, pH 7.2-7.4
Reagent Diluent: (Catalog # DY995), or 1% BSA in PBS, pH 7.2-7.4, 0.2 µm filtered
Substrate Solution: 1:1 mixture of Color Reagent A (H2O2) and Color Reagent B (Tetramethylbenzidine) (Catalog # DY999)
Stop Solution: 2 N H2SO4 (Catalog # DY994)
Microplates: R&D Systems (Catalog # DY990)
Plate Sealers: ELISA Plate Sealers (Catalog # DY992)
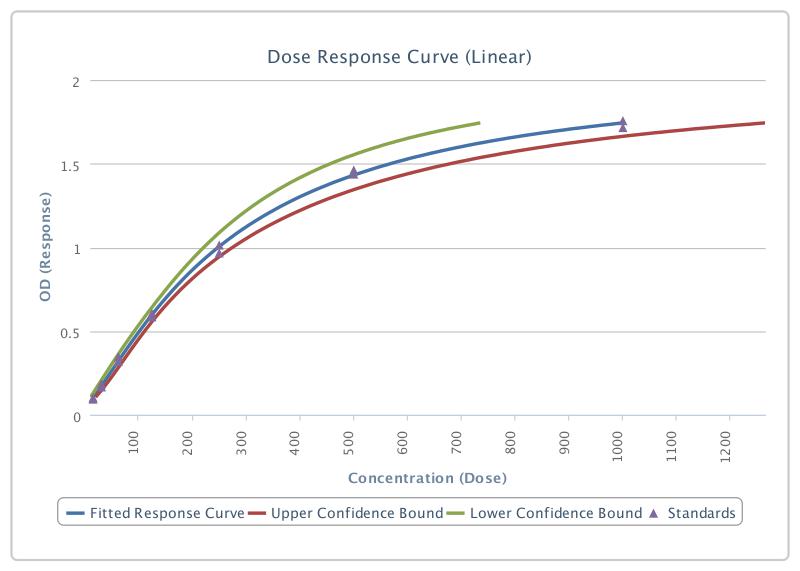
Scientific Data
Product Datasheets
Preparation and Storage
Background: VEGF
Vascular endothelial growth factor (VEGF or VEGF-A), also known as vascular permeability factor (VPF), is a potent mediator of both angiogenesis and vasculogenesis in the fetus and in adults (1-3). It is a member of the PDGF family that is characterized by the presence of eight conserved cysteine residues in a cystine knot structure and the formation of anti-parallel disulfide-linked dimers (4). Alternately spliced isoforms of 120, 164 and 188 amino acids (aa) have been found in mice, while 121, 145, 165, 183, 189, and 206 aa isoforms have been identified in humans (2, 4). In humans, VEGF165 appears to be the most abundant and potent isoform, followed by VEGF121 and VEGF189 (3, 4). The same pattern may exist in mice. Isoforms other than VEGF120 and VEGF121 contain basic heparin-binding regions and are not freely diffusible (4). Mouse VEGF164 shares 97% aa sequence identity with corresponding regions of rat VEGF. It also shares 89% aa sequence identity with human and porcine VEGF, 88% with bovine VEGF, and 90% with feline, equine, and canine VEGF. VEGF is expressed in multiple cells and tissues including skeletal and cardiac muscle (5, 6), hepatocytes (7), osteoblasts (8), neutrophils (9), macrophages (10), keratinocytes (11), brown adipose tissue (12), CD34+ stem cells (13), endothelial cells (14), fibroblasts, and vascular smooth muscle cells (15). VEGF expression is induced by hypoxia and cytokines such as IL-1, IL-6, IL-8, Oncostatin M, and TNF-alpha (3, 4, 9, 16). The isoforms are differentially expressed during development and in the adult (3).
Assay Procedure
GENERAL ELISA PROTOCOL
Plate Preparation
- Dilute the Capture Antibody to the working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL per well of the diluted Capture Antibody. Seal the plate and incubate overnight at room temperature.
- Aspirate each well and wash with Wash Buffer, repeating the process two times for a total of three washes. Wash by filling each well with Wash Buffer (400 μL) using a squirt bottle, manifold dispenser, or autowasher. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any remaining Wash Buffer by aspirating or by inverting the plate and blotting it against clean paper towels.
- Block plates by adding 300 μL Reagent Diluent to each well. Incubate at room temperature for a minimum of 1 hour.
- Repeat the aspiration/wash as in step 2. The plates are now ready for sample addition.
Assay Procedure
- Add 100 μL of sample or standards in Reagent Diluent, or an appropriate diluent, per well. Cover with an adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the Detection Antibody, diluted in Reagent Diluent, to each well. Cover with a new adhesive strip and incubate 2 hours at room temperature.
- Repeat the aspiration/wash as in step 2 of Plate Preparation.
- Add 100 μL of the working dilution of Streptavidin-HRP to each well. Cover the plate and incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Repeat the aspiration/wash as in step 2.
- Add 100 μL of Substrate Solution to each well. Incubate for 20 minutes at room temperature. Avoid placing the plate in direct light.
- Add 50 μL of Stop Solution to each well. Gently tap the plate to ensure thorough mixing.
- Determine the optical density of each well immediately, using a microplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. If wavelength correction is not available, subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.
Citations for Mouse VEGF DuoSet ELISA
R&D Systems personnel manually curate a database that contains references using R&D Systems products. The data collected includes not only links to publications in PubMed, but also provides information about sample types, species, and experimental conditions.
63
Citations: Showing 1 - 10
Filter your results:
Filter by:
-
Myeloid PHD2 Conditional Knockout Improves Intraplaque Angiogenesis and Vascular Remodeling in a Murine Model of Venous Bypass Grafting
Authors: Sluiter, TJ;Tillie, RJHA;de Jong, A;de Bruijn, JBG;Peters, HAB;van de Leijgraaf, R;Halawani, R;Westmaas, M;Starink, LIW;Quax, PHA;Sluimer, JC;de Vries, MR;
Journal of the American Heart Association
Species: Transgenic Mouse
Sample Types: Cell Culture Supernates
-
Signaling events at TMEM doorways provide potential targets for inhibiting breast cancer dissemination
Authors: Surve, CR;Duran, CL;Ye, X;Chen, X;Lin, Y;Harney, AS;Wang, Y;Sharma, VP;Stanley, ER;McAuliffe, JC;Entenberg, D;Oktay, MH;Condeelis, JS;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Cell Culture Supernates
-
Microporous Hydroxyapatite-Based Ceramics Alter the Physiology of Endothelial Cells through Physical and Chemical Cues
Authors: Usseglio, J;Dumur, A;Pagès, E;Renaudie, É;Abélanet, A;Brie, J;Champion, É;Magnaudeix, A;
Journal of functional biomaterials
Species: Mouse
Sample Types: Cell Culture Supernates
-
Chromatin accessibility uncovers KRAS-driven FOSL2 promoting pancreatic ductal adenocarcinoma progression through up-regulation of CCL28
Authors: Zhang, S;Li, P;Li, J;Gao, J;Qi, Q;Dong, G;Liu, X;Jiao, Q;Wang, Y;Du, L;Zhan, H;Xu, S;Wang, C;
British journal of cancer
Species: Mouse
Sample Types: Serum
-
Neural stem and progenitor cells support and protect adult hippocampal function via vascular endothelial growth factor secretion
Authors: Denninger, JK;Miller, LN;Walters, AE;Hosawi, M;Sebring, G;Rieskamp, JD;Ding, T;Rindani, R;Chen, KS;Senthilvelan, S;Volk, A;Zhao, F;Askwith, C;Kirby, ED;
bioRxiv : the preprint server for biology
Species: Mouse
Sample Types: Tissue Homogenates
-
Knocking-Down CD147/EMMPRIN Expression in CT26 Colon Carcinoma Forces the Cells into Cellular and Angiogenic Dormancy That Can Be Reversed by Interactions with Macrophages
Authors: G Feigelman, E Simanovich, P Brockmeyer, MA Rahat
Biomedicines, 2023-03-02;11(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Finerenone, a Non-Steroidal Mineralocorticoid Receptor Antagonist, Reduces Vascular Injury and Increases Regulatory T-Cells: Studies in Rodents with Diabetic and Neovascular Retinopathy
Authors: JR Jerome, D Deliyanti, V Suphapimol, P Kolkhof, JL Wilkinson-
International Journal of Molecular Sciences, 2023-01-25;24(3):.
Species: Mouse
Sample Types: Cell Lysates
-
Changes in adenosine receptors and neurotrophic factors in the SOD1G93A mouse model of amyotrophic lateral sclerosis: Modulation by chronic caffeine
Authors: N Rei, CA Valente, SH Vaz, M Farinha-Fe, JA Ribeiro, AM Sebastião
PLoS ONE, 2022-12-14;17(12):e0272104.
Species: Mouse
Sample Types: Tissue Homogenates
-
KDM6A Loss Recruits Tumor-Associated Neutrophils and Promotes Neutrophil Extracellular Trap Formation in Pancreatic Cancer
Authors: J Yang, L Jin, HS Kim, F Tian, Z Yi, K Bedi, M Ljungman, M Pasca di M, H Crawford, J Shi
Cancer Research, 2022-11-15;0(0):OF1-OF14.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Bactericidal/Permeability-Increasing Protein Downregulates the Inflammatory Response in In Vivo Models of Arthritis
Authors: A Scanu, R Luisetto, F Oliviero, F Galuppini, V Lazzarin, G Pennelli, S Masiero, L Punzi
International Journal of Molecular Sciences, 2022-10-28;23(21):.
Species: Mouse
Sample Types: Serum
-
Mild dyslipidemia accelerates tumorigenesis through expansion of Ly6Chi monocytes and differentiation to pro-angiogenic myeloid cells
Authors: T Tran, JR Lavillegra, C Lereverend, B Esposito, L Cartier, M Montabord, J Tran-Rajau, M Diedisheim, N Gruel, K Ouguerram, L Paolini, O Lenoir, E Pinteaux, E Brabencova, C Tanchot, P Urquia, J Lehmann-Ch, R Le Naour, Y Merrouche, C Stockmann, Z Mallat, A Tedgui, H Ait-Oufell, E Tartour, S Potteaux
Nature Communications, 2022-09-14;13(1):5399.
Species: Mouse
Sample Types: Tissue Homogenates
-
Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells
Authors: M Llorián-Sa, EM Byrne, M Szczepan, K Little, M Chen, H Xu
Journal of Neuroinflammation, 2022-07-14;19(1):182.
Species: Mouse
Sample Types: Cell Culture Supernates
-
VEGF-Mediated Augmentation of Autophagic and Lysosomal Activity in Endothelial Cells Defends against Intracellular Streptococcus pyogenes
Authors: SL Lu, H Omori, Y Zhou, YS Lin, CC Liu, JJ Wu, T Noda
MBio, 2022-07-05;0(0):e0123322.
Species: Mouse
Sample Types: Serum
-
Sirtuin 1 Induces Choroidal Neovascularization and Triggers Age-Related Macular Degeneration by Promoting LCN2 through SOX9 Deacetylation
Authors: S Zhao, Z Huang, H Jiang, J Xiu, L Zhang, Q Long, Y Yang, L Yu, L Lu, H Gu
Oncogene, 2022-06-09;2022(0):1671438.
Species: Mouse
Sample Types: Tissue Homogenates
-
Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression
Authors: H Matsuno, S Tsuchimine, K O'Hashi, K Sakai, K Hattori, S Hidese, S Nakajima, S Chiba, A Yoshimura, N Fukuzato, M Kando, M Tatsumi, S Ogawa, N Ichinohe, H Kunugi, K Sohya
Molecular Psychiatry, 2022-05-26;0(0):.
Species: Mouse
Sample Types: Serum
-
Kinetics of LYVE-1-positive M2-like macrophages in developing and repairing dental pulp in vivo and their pro-angiogenic activity in vitro
Authors: TQ Kieu, K Tazawa, N Kawashima, S Noda, M Fujii, K Nara, K Hashimoto, P Han, T Okiji
Scientific Reports, 2022-03-25;12(1):5176.
Species: Mouse
Sample Types: Conditioned Media
-
Macrophage IL-1beta promotes arteriogenesis by autocrine STAT3- and NF-kappaB-mediated transcription of pro-angiogenic VEGF-A
Authors: CS Mantsounga, C Lee, J Neverson, S Sharma, A Healy, JM Berus, C Parry, NM Ceneri, F López-Girá, HJ Chun, Q Lu, F Sellke, G Choudhary, AR Morrison
Cell Reports, 2022-02-01;38(5):110309.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization
Authors: L Huang, W Liang, K Zhou, RA Wassel, ZD Ridge, JX Ma, B Wang
Biology, 2021-12-15;10(12):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Intramuscular Exposure to a Lethal Dose of Ricin Toxin Leads to Endothelial Glycocalyx Shedding and Microvascular Flow Abnormality in Mice and Swine
Authors: A Sapoznikov, Y Gal, Y Evgy, M Aftalion, S Katalan, T Sabo, C Kronman, R Falach
International Journal of Molecular Sciences, 2021-11-16;22(22):.
Species: Mouse
Sample Types: Serum
-
Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation
Authors: H Shokirova, T Inomata, T Saitoh, J Zhu, K Fujio, Y Okumura, A Yanagawa, K Fujimoto, J Sung, A Eguchi, M Miura, K Nagino, K Hirosawa, M Kuwahara, Y Akasaki, H Nagase, A Murakami
Scientific Reports, 2021-04-21;11(1):8647.
Species: Mouse
Sample Types: Tissue Homogenates
-
VEGF Contributes to Mesenchymal Stem Cell-Mediated Reversion of Nor1-Dependent Hypertrophy in iPS Cell-Derived Cardiomyocytes
Authors: D Philipp, M Holthaus, V Basoah, K Pfannkuche, L Suhr, T Wahlers, A Paunel-Gör
Stem Cells International, 2021-04-10;2021(0):8888575.
Species: Mouse
Sample Types: Cell Lysates
-
Nanotransfection-based vasculogenic cell reprogramming drives functional recovery in a mouse model of ischemic stroke
Authors: LR Lemmerman, MHH Balch, JT Moore, D Alzate-Cor, MA Rincon-Ben, A Salazar-Pu, S Gnyawali, HN Harris, W Lawrence, L Ortega-Pin, L Wilch, IB Risser, AJ Maxwell, S Duarte-San, D Dodd, GP Guio-Vega, DM McTigue, WD Arnold, SM Nimjee, CK Sen, S Khanna, C Rink, N Higuita-Ca, D Gallego-Pe
Science Advances, 2021-03-19;7(12):.
Species: Mouse
Sample Types: Cell Lysates
-
The NFIB-ERO1A axis promotes breast cancer metastatic colonization of disseminated tumour cells
Authors: F Zilli, P Marques Ra, P Auf der Ma, C Jehanno, A Sethi, MM Coissieux, T Eichlisber, L Sauteur, A Rouchon, L Bonapace, J Pinto Cout, R Rad, MR Jensen, A Banfi, MB Stadler, M Bentires-A
Embo Molecular Medicine, 2021-03-10;0(0):e13162.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Inhibitory effect of ginsenoside Rg3 on cancer stemness and mesenchymal transition in breast cancer via regulation of myeloid-derived suppressor cells
Authors: JH Song, DY Eum, SY Park, YH Jin, JW Shim, SJ Park, MY Kim, SJ Park, K Heo, YJ Choi
PLoS ONE, 2020-10-22;15(10):e0240533.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Recombinant Myxoma Virus-Derived Immune Modulator M-T7 Accelerates Cutaneous Wound Healing and Improves Tissue Remodeling
Authors: JR Yaron, L Zhang, Q Guo, EA Awo, M Burgin, LN Schutz, N Zhang, J Kilbourne, J Daggett-Vo, KM Lowe, AR Lucas
Pharmaceutics, 2020-10-22;12(11):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Ischemia-Like Stress Conditions Stimulate Trophic Activities of Adipose-Derived Stromal/Stem Cells
Authors: J Bachmann, E Ehlert, M Becker, C Otto, K Radeloff, T Blunk, P Bauer-Krei
Cells, 2020-08-21;9(9):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye
Authors: A Wolf, M Herb, M Schramm, T Langmann
Nat Commun, 2020-06-01;11(1):2709.
Species: Mouse
Sample Types: Tissue Homogenates
-
Protein Kinase A Catalytic Subunit Is a Molecular Switch that Promotes the Pro-tumoral Function of Macrophages
Authors: YR Na, JW Kwon, DY Kim, H Chung, J Song, D Jung, H Quan, D Kim, JS Kim, YW Ju, W Han, HS Ryu, YS Lee, JJ Hong, SH Seok
Cell Rep, 2020-05-12;31(6):107643.
Species: Mouse
Sample Types:
-
Functional Evaluation of AMD-Associated Risk Variants of Complement Factor B
Authors: C Pilotti, J Greenwood, SE Moss
Invest. Ophthalmol. Vis. Sci., 2020-05-11;61(5):19.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Different Frequency of Cyclic Tensile Strain Relates to Anabolic/Catabolic Conditions Consistent with Immunohistochemical Staining Intensity in Tenocytes
Authors: Y Kubo, B Hoffmann, K Goltz, U Schnakenbe, H Jahr, R Merkel, G Schulze-Ta, T Pufe, M Tohidnezha
Int J Mol Sci, 2020-02-06;21(3):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models
Authors: R Li, Y Huang, J Lin
Nat Commun, 2020-01-31;11(1):642.
Species: Mouse
Sample Types: Serum
-
ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer's disease.
Authors: Evans C, Miners J, Piva G, Willis C, Heard D, Kidd E, Good M, Kehoe P
Acta Neuropathol, 2020-01-25;139(3):485-502.
Species: Mouse
Sample Types: Cell Lysates
-
Inhibition of FLT1 ameliorates muscular dystrophy phenotype by increased vasculature in a mouse model of Duchenne muscular dystrophy
Authors: M Verma, Y Shimizu-Mo, Y Asakura, JP Ennen, J Bosco, Z Zhou, GH Fong, S Josiah, D Keefe, A Asakura
PLoS Genet., 2019-12-26;15(12):e1008468.
Species: Mouse
Sample Types: Cell Culture Su
-
A Virus-Derived Immune Modulating Serpin Accelerates Wound Closure with Improved Collagen Remodeling
Authors: L Zhang, JR Yaron, AM Tafoya, SE Wallace, J Kilbourne, S Haydel, K Rege, G McFadden, AR Lucas
J Clin Med, 2019-10-04;8(10):.
Species: Mouse
Sample Types: Tissue Homogenates
-
Oral administration of EPA-rich oil impairs collagen reorganization due to elevated production of IL-10 during skin wound healing in mice
Authors: B Burger, CMC Kühl, T Candreva, RDS Cardoso, JR Silva, BG Castelucci, SR Consonni, HL Fisk, PC Calder, MAR Vinolo, HG Rodrigues
Sci Rep, 2019-06-24;9(1):9119.
Species: Mouse
Sample Types: Tissue Homogenates
-
Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling
Authors: M Verma, Y Asakura, BSR Murakonda, T Pengo, C Latroche, B Chazaud, LK McLoon, A Asakura
Cell Stem Cell, 2018-10-04;23(4):530-543.e9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
SOCS3 deficiency in myeloid cells promotes retinal degeneration and angiogenesis through arginase-1 up-regulation in experimental autoimmune uveoretinitis
Authors: M Chen, J Zhao, IH Ali, S Marry, J Augustine, M Bhuckory, A Lynch, A Kissenpfen, H Xu
Am. J. Pathol., 2018-02-13;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Proangiogenic alginate-g-pyrrole hydrogel with decoupled control of mechanical rigidity and electrically conductivity
Authors: RJ DeVolder, Y Seo, H Kong
Biomater Res, 2017-11-07;21(0):24.
Species: Mouse
Sample Types: Whole Cells
-
MCPIP1 downregulation in clear cell renal cell carcinoma promotes vascularization and metastatic progression
Authors: P Marona, J Górka, Z Mazurek, W Wilk, J Rys, M Majka, J Jura, K Miekus
Cancer Res., 2017-07-17;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
ECM-based macroporous sponges release essential factors to support the growth of hematopoietic cells
J Control Release, 2016-09-23;0(0):.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Interactions among Lung Cancer Cells, Fibroblasts, and Macrophages in 3D Co-Cultures and the Impact on MMP-1 and VEGF Expression
Authors: Xiao-Qing Liu
PLoS ONE, 2016-05-27;11(5):e0156268.
Species: Human
Sample Types: Cell Culture Supernates
-
Tumor Microenvironment Remodeling by 4-Methylumbelliferone Boosts the Antitumor Effect of Combined Immunotherapy in Murine Colorectal Carcinoma.
Authors: Malvicini M, Fiore E, Ghiaccio V, Piccioni F, Rizzo M, Olmedo Bonadeo L, Garcia M, Rodriguez M, Bayo J, Peixoto E, Atorrasagasti C, Alaniz L, Aquino J, Matar P, Mazzolini G
Mol Ther, 2015-06-24;23(9):1444-55.
Species: Mouse
Sample Types: Serum
-
VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors.
Authors: Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet A, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M
J Exp Med, 2015-01-19;212(2):139-48.
Species: Mouse
Sample Types: Tissue Homogenates
-
FcgammaRIIb inhibits immune complex-induced VEGF-A production and intranodal lymphangiogenesis.
Authors: Clatworthy M, Harford S, Mathews R, Smith K
Proc Natl Acad Sci U S A, 2014-12-04;111(50):17971-6.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis.
Authors: Bonapace L, Coissieux M, Wyckoff J, Mertz K, Varga Z, Junt T, Bentires-Alj M
Nature, 2014-10-22;515(7525):130-3.
Species: Mouse
Sample Types: Tissue Homogenates
-
Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors.
Authors: Ryzhov S, Pickup M, Chytil A, Gorska A, Zhang Q, Owens P, Feoktistov I, Moses H, Novitskiy S
J Immunol, 2014-08-15;193(6):3155-64.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Advances in the pathophysiology of pre-eclampsia and related podocyte injury.
Authors: Craici, Iasmina, Wagner, Steven J, Weissgerber, Tracey L, Grande, Joseph P, Garovic, Vesna D
Kidney Int, 2014-02-26;86(2):275-85.
Species: Rat
Sample Types:
-
The monocyte to macrophage transition in the murine sterile wound.
Authors: Crane, Meredith, Daley, Jean M, van Houtte, Olivier, Brancato, Samielle, Henry, William, Albina, Jorge E
PLoS ONE, 2014-01-22;9(1):e86660.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation.
Authors: Csoka B, Koscso B, Toro G, Kokai E, Virag L, Nemeth Z, Pacher P, Bai P, Hasko G
Diabetes, 2013-11-05;63(3):850-66.
Species: Mouse
Sample Types: Plasma
-
The role of IL-1beta in the early tumor cell-induced angiogenic response.
Authors: Carmi Y, Dotan S, Rider P, Kaplanov I, White M, Baron R, Abutbul S, Huszar M, Dinarello C, Apte R, Voronov E
J Immunol, 2013-03-08;190(7):3500-9.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Von Hippel-Lindau protein in the RPE is essential for normal ocular growth and vascular development.
Authors: Lange CA, Luhmann UF, Mowat FM
Development, 2012-05-23;139(13):2340-50.
Species: Mouse
Sample Types: Tissue Homogenates
-
Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells.
Authors: Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G
Nature, 2011-07-13;475(7355):226-30.
Species: Mouse
Sample Types: Ascites Fluid
-
Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex.
Authors: Howell KR, Kutiyanawalla A, Pillai A
PLoS ONE, 2011-05-27;6(5):e20198.
Species: Mouse
Sample Types: Serum
-
CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes.
Authors: Tzeng TC, Chyou S, Tian S, Webster B, Carpenter AC, Guaiquil VH, Lu TT
J. Immunol., 2010-03-15;184(8):4247-57.
Species: Mouse
Sample Types: Tissue Homogenates
-
IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart.
Authors: Banerjee I, Fuseler JW, Intwala AR, Baudino TA
Am. J. Physiol. Heart Circ. Physiol., 2009-02-20;296(5):H1694-704.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia.
Authors: Lukkarinen H, Hogmalm A, Lappalainen U, Bry K
Am. J. Respir. Cell Mol. Biol., 2008-12-18;41(1):59-68.
Species: Mouse
Sample Types: Tissue Homogenates
-
CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process.
Authors: Wu Y, Li YY, Matsushima K, Baba T, Mukaida N
J. Immunol., 2008-11-01;181(9):6384-93.
Species: Mouse
Sample Types: Tissue Homogenates
-
Fibroblast-type reticular stromal cells regulate the lymph node vasculature.
Authors: Chyou S, Ekland EH, Carpenter AC, Tzeng TC, Tian S, Michaud M, Madri JA, Lu TT
J. Immunol., 2008-09-15;181(6):3887-96.
Species: Mouse
Sample Types: Tissue Homogenates
-
Adenosine receptors in regulation of dendritic cell differentiation and function.
Authors: Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM
Blood, 2008-06-17;112(5):1822-31.
Species: Mouse
Sample Types: Cell Culture Supernates
-
In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power.
Authors: Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR
Surgery, 2007-08-01;142(2):215-21.
Species: Mouse
Sample Types: Cell Culture Supernates
-
A functional comparison of canine and murine bone marrow derived cultured mast cells.
Authors: Lin TY, London CA
Vet. Immunol. Immunopathol., 2006-10-06;114(3):320-34.
Species: Mouse
Sample Types: Cell Culture Supernates
-
Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1.
Authors: Barcelos LS, Talvani A, Teixeira AS, Vieira LQ, Cassali GD, Andrade SP, Teixeira MM
J. Leukoc. Biol., 2005-05-13;78(2):352-8.
Species: Mouse
Sample Types: Complex Sample Type
-
Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells.
Authors: Noel D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, Millet V, Turgeman G, Perricaudet M, Sany J, Jorgensen C
Stem Cells, 2004-01-01;22(1):74-85.
Species: Mouse
Sample Types: Cell Culture Supernates
FAQs
No product specific FAQs exist for this product, however you may
View all ELISA FAQsReviews for Mouse VEGF DuoSet ELISA
Average Rating: 5 (Based on 3 Reviews)
Have you used Mouse VEGF DuoSet ELISA?
Submit a review and receive an Amazon gift card.
$25/€18/£15/$25CAN/¥75 Yuan/¥2500 Yen for a review with an image
$10/€7/£6/$10 CAD/¥70 Yuan/¥1110 Yen for a review without an image
Filter by:
Worked perfectly for standards, my samples and a control of diluted recombinant VEGF. Excellent elisa and easy to use